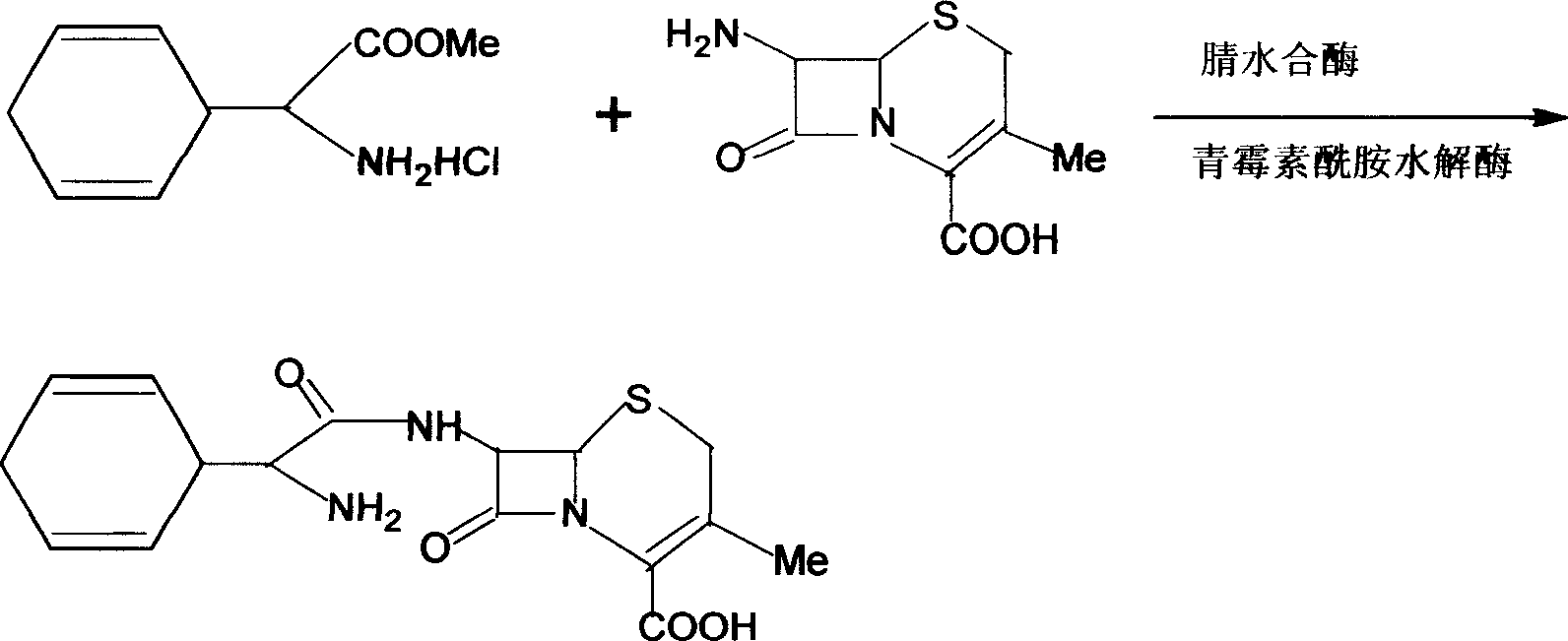
Cefradine preparing process
A cephradine and reaction technology, applied in the field of biopharmaceuticals, can solve the problems that the catalytic performance is easily affected by pH value, temperature, ionic strength and organic solvents, the performance of the catalyst cannot be maximized, and the price of the biocatalyst is expensive. Effects of separation, less solvent, and reduced sewage discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve dihydrophenylglycine methyl ester and 7-ADCA in a ratio of 1:1 in a reaction vessel equipped with a two-phase system, add 4mol / L sulfuric acid to adjust the pH value of the system to 5.0, and use a temperature control device to cool the reaction solution The temperature is controlled at T=5°C, and immobilized penicillin acylase (carrier is polypropylene fiber, acetate fiber, amino silica gel, resin, diatomaceous earth) and nitrile hydratase are added for about 1 hour, and they are carried out under the following conditions Acylation: temperature T = 15°C, pH = 6.5, continuous reaction for about 3 hours, the results of sample analysis showed that 65% of 7-ADCA had been converted into cephradine. At this stage, samples are taken from the upper and lower phases every half hour, and the concentrations of reactants and products are analyzed by HPLC. After the last detection, the centrifugal device is rotated to separate the upper and lower phases again, and the upper...
Embodiment 2
[0034] Dissolve dihydrophenylglycine methyl ester and 7-ADCA in a ratio of 1:1 in a reaction vessel equipped with a two-phase system, add 4mol / L sulfuric acid to adjust the pH value of the system to 6.5, and use a temperature control device to cool the reaction solution The temperature is controlled at T=20°C, and immobilized penicillin acylase (carrier is polypropylene fiber, acetate fiber, amino silica gel, resin, diatomaceous earth) and nitrile hydratase are added for about 1 hour, and they are carried out under the following conditions Acylation: temperature T = 15°C, pH = 6.5, continuous reaction for about 3 hours, the results of sample analysis showed that 70% of 7-ADCA had been converted into cephradine. At this stage, samples are taken from the upper and lower phases every half hour, and the concentrations of reactants and products are analyzed by HPLC. After the last detection, the centrifugal device is rotated to separate the upper and lower phases again, and the uppe...
Embodiment 3
[0036] Dissolve dihydrophenylglycine methyl ester and 7-ADCA in a ratio of 1.5:1 in a reaction vessel equipped with a two-phase system, add 4mol / L sulfuric acid to adjust the pH value of the system to 6.5, and use a temperature control device to cool the reaction solution The temperature is controlled at T=10°C, and immobilized penicillin acylase (carrier is polypropylene fiber, acetate fiber, amino silica gel, resin, diatomaceous earth) and nitrile hydratase are added for about 1 hour, and they are carried out under the following conditions Acylation: temperature T = 15°C, pH = 6.5, continuous reaction for about 3 hours, the results of sample analysis showed that 75% of 7-ADCA had been converted into cephradine. At this stage, samples are taken from the upper and lower phases every half hour, and the concentrations of reactants and products are analyzed by HPLC. After the last detection, the centrifugal device is rotated to separate the upper and lower phases again, and the up...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com