Aromatic substitution spiro indolyl diketopiperazine compound and synthesis method thereof
A technology for indole diketopiperazines and synthesis methods, which is applied in the field of spiro indole diketopiperazines substituted by aromatic rings and their synthesis, and can solve the problems of poor stereoselectivity, low yield, cumbersome post-treatment, etc. , to achieve the effect of reducing reaction steps, saving costs and improving final yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
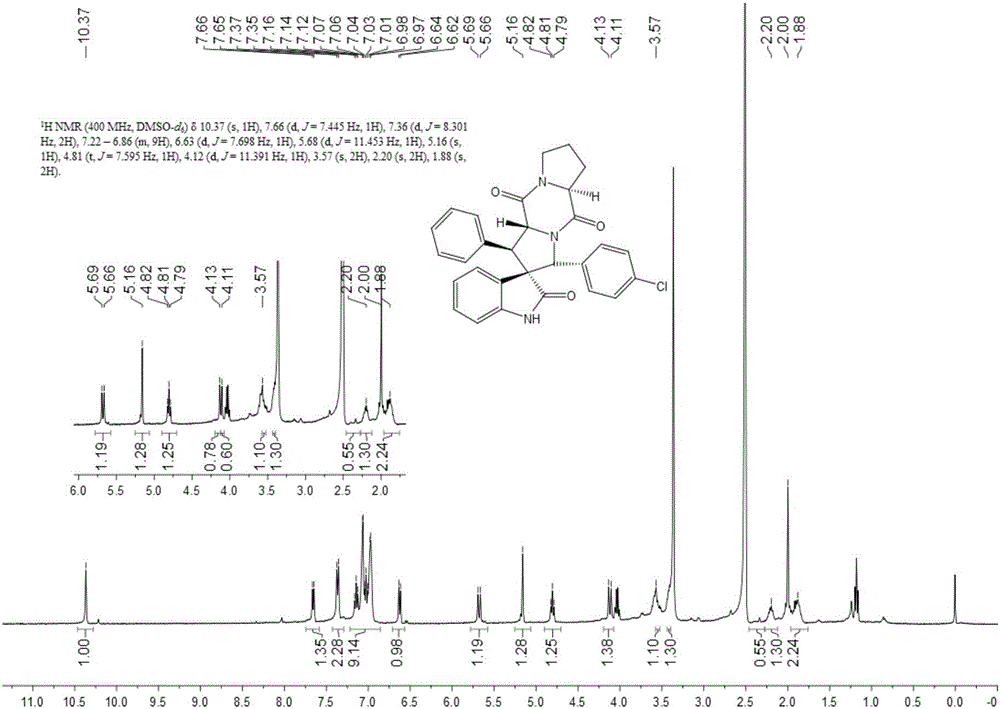
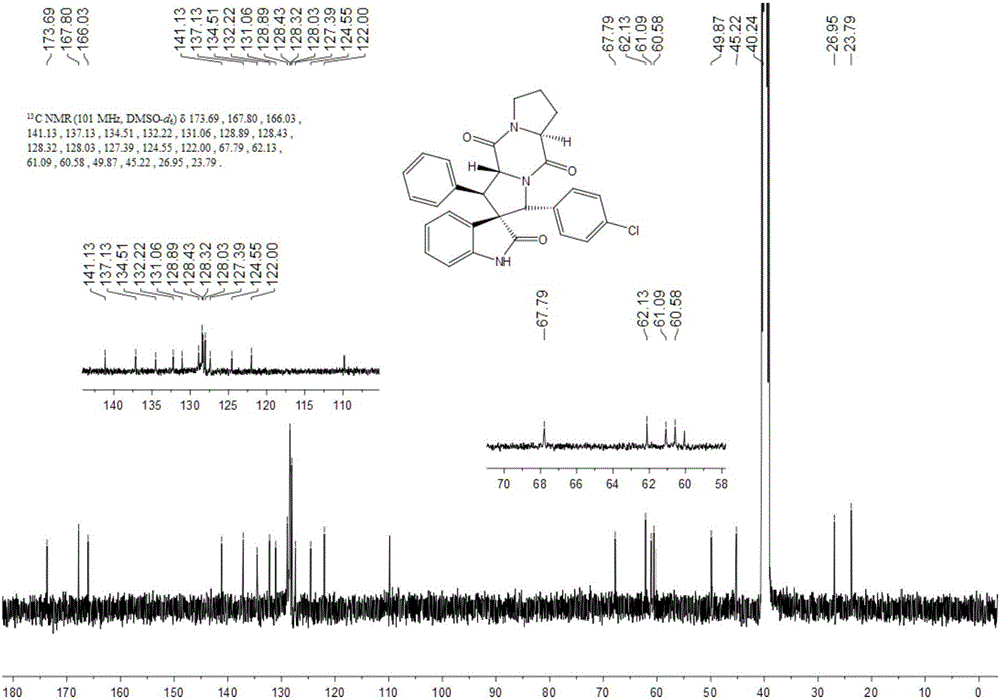
Embodiment 1
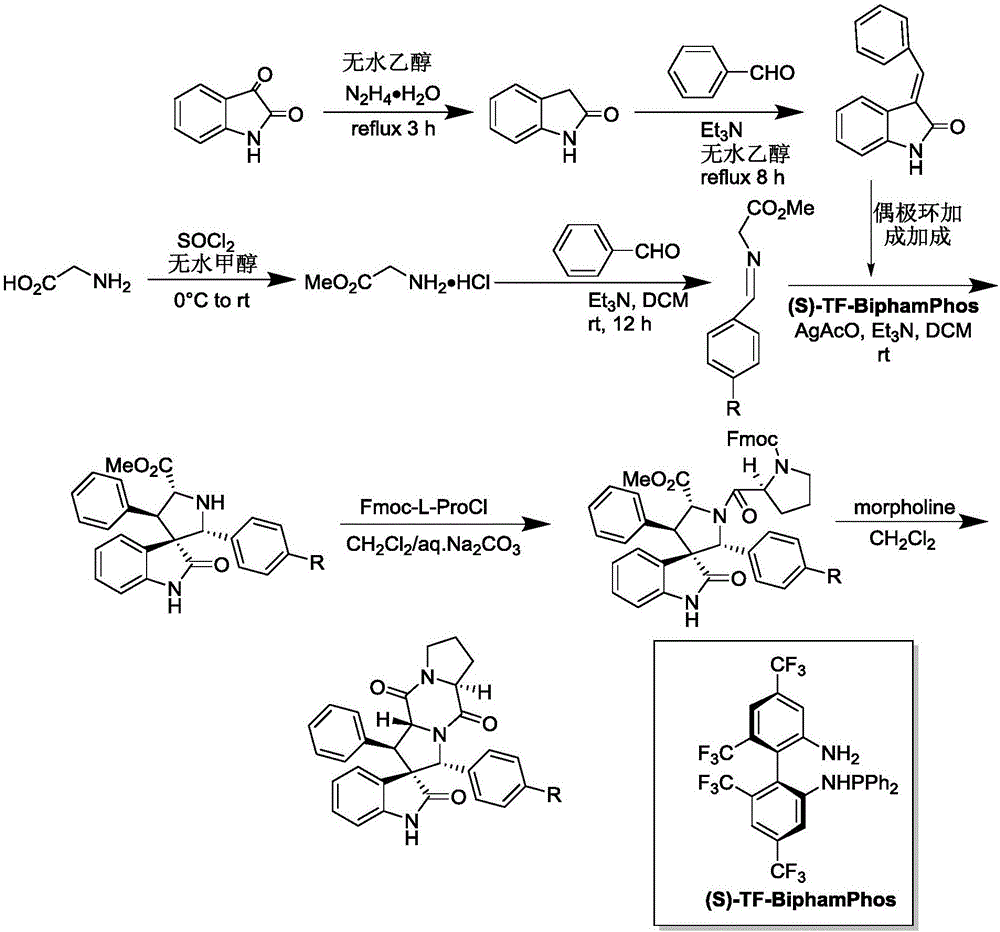
[0041] 1) Add 50ml of absolute ethanol as a solvent to a 100ml single-necked flask equipped with a condensation reflux device, then add 34mmol of isatin and 40mmol of hydrazine hydrate to reflux for 3h, cool to 0°C, add 102mmol of sodium hydroxide, reflux for 0.5h, and wait for cooling After reaching room temperature, add 100ml of water, adjust the Ph to 2 with 2mol / L hydrochloric acid, combine the organic phases after liquid separation and extraction, concentrate under reduced pressure and directly have a pink solid precipitate out, and obtain a white solid dihydrogen after recrystallization from absolute ethanol Indole-2-one.
[0042] 2) Add 15ml of absolute ethanol as a solvent in a 50ml single-necked flask equipped with a condensation reflux device, then add 10mmol indolin-2 ketone, 12mmol benzaldehyde and 1mmol piperidine, reflux, TLC detects the progress of the reaction, when the reaction When carried out to 8h, TLC detects that the raw material point of indolin-2 ketone...
Embodiment 2
[0051] 1) Add 50ml of absolute ethanol as a solvent to a 100ml single-necked flask equipped with a condensation reflux device, then add 34mmol of isatin and 40mmol of hydrazine hydrate to reflux for 3h, cool to 0°C, add 102mmol of sodium hydroxide, reflux for 0.5h, and wait for cooling After reaching room temperature, add 100ml of water, adjust the Ph to 2 with 2mol / L hydrochloric acid, combine the organic phases after liquid separation and extraction, concentrate under reduced pressure and directly have a pink solid precipitate out, and obtain a white solid dihydrogen after recrystallization from absolute ethanol Indole-2-one.
[0052] 2) Add 15ml of absolute ethanol as solvent in a 50ml single-necked flask equipped with a condensation reflux device, then add 10mmol indolin-2 ketone, 10mmol benzaldehyde and 1mmol piperidine, reflux, TLC detects the progress of the reaction, when the reaction When carried out to 8h, TLC detects that the raw material point of indolin-2 ketone d...
Embodiment 3
[0061] 1) Add 50ml of absolute ethanol as a solvent to a 100ml single-necked flask equipped with a condensation reflux device, then add 34mmol of isatin and 40mmol of hydrazine hydrate to reflux for 3h, cool to 0°C, add 102mmol of sodium hydroxide, reflux for 0.5h, and wait for cooling After reaching room temperature, add 100ml of water, adjust the Ph to 2 with 2mol / L hydrochloric acid, combine the organic phases after liquid separation and extraction, concentrate under reduced pressure and directly have a pink solid precipitate out, and obtain a white solid dihydrogen after recrystallization from absolute ethanol Indole-2-one.
[0062] 2) Add 15ml of absolute ethanol as a solvent in a 50ml single-necked flask equipped with a condensing reflux device, then add 10mmol indolin-2 ketone, 11mmol benzaldehyde and 1mmol piperidine, reflux, TLC detects the progress of the reaction, when the reaction When carried out to 8h, TLC detects that the raw material point of indolin-2 ketone d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com