Feather weight synthesis method of Fmoc-PNA-T-OH
A fmoc-pna-t-oh, kilogram-level technology, applied in the field of PNA synthesis, can solve the problems of restricting PNA application research work, small synthesis scale, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The present invention will be described in detail below in conjunction with the embodiments.
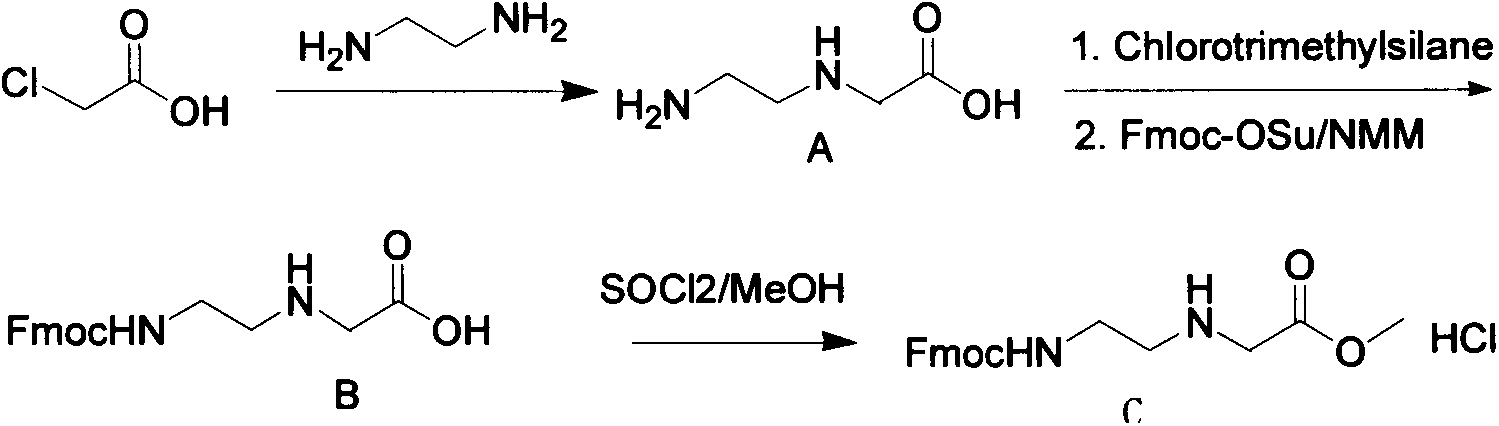
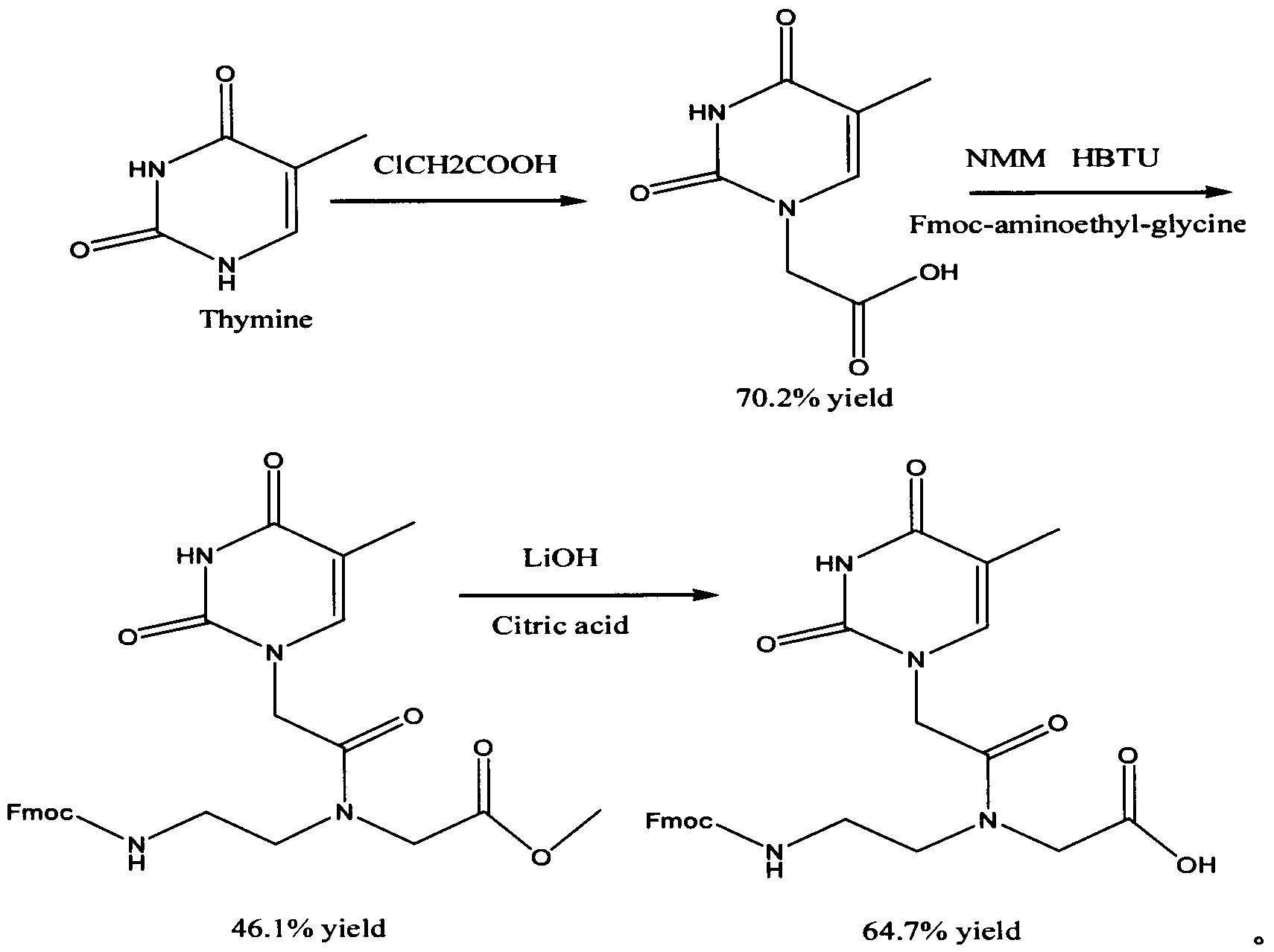
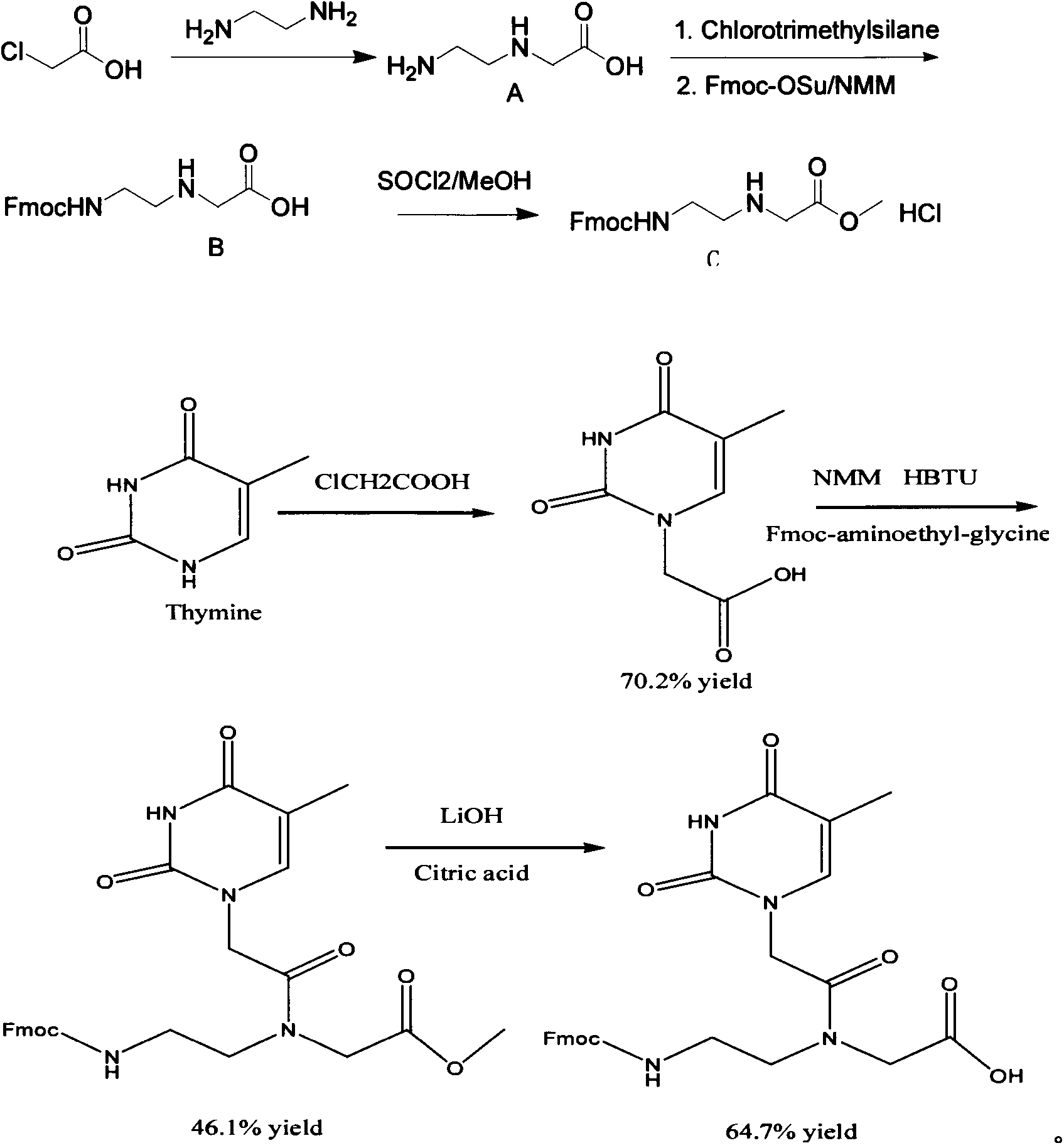
[0021] A kilogram-level synthesis of Fmoc-PNA-T-OH method includes the following steps:
[0022] Step 1) 1 liter three-necked flask is filled with 500 ml of ethylene diamine, cooled to 10 degrees in an ice bath, and 75 g of chloroacetic acid is added in batches to control the temperature below 10 degrees. After each batch is added, observe that it is completely consumed before adding One batch, after the addition is complete, react overnight at room temperature, spin-dry the ethylenediamine, recover the ethylenediamine for recycling, use an oil pump to remove the ethylenediamine as much as possible, and recrystallize with DMSO to obtain white powdery crystals, which are N- (2-Aminoethyl)glycine, the yield is 40-50%;
[0023] Step 2) Dissolve 50g of N-(2-aminoethyl)glycine in a mixed solution of 850ml of dichloromethane and 50ml of N,N-dimethylformamide, add 134ml of trimethylchloros...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com