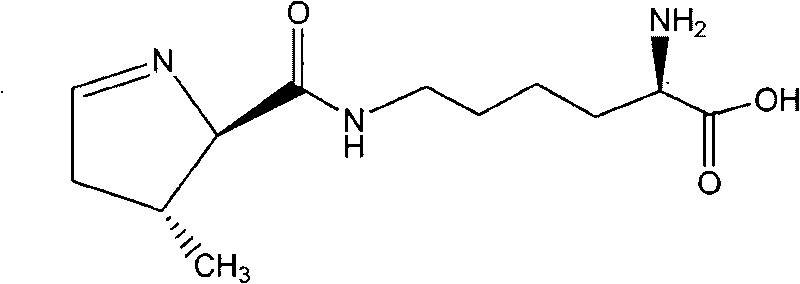
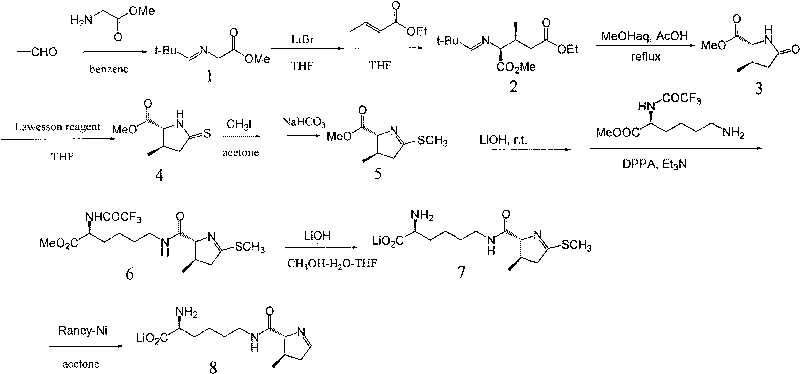
Chemical complete synthesis method for 22nd natural amino acid-pyrrolysine
A technology of pyrrolysine and natural amino acid, applied in the directions of organic chemistry, bulk chemical production, etc., can solve the problems of instability and change of imine bonds, and achieve the effects of simple reaction device, low operating cost, and not easy to deteriorate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. Methyl (E)-2-(2,2-dimethylpropyleneamino)acetate (1)
[0044] Add 0.740g glycine methyl ester hydrochloride (0.578mmol), 8ml benzene, 0.507g 2,2-dimethylpropanal (0.578mmol, 0.64ml) into a 25ml flask, then drop into 0.584g triethylamine (0.578 mmol), start heating, and reflux for 1h. After the reaction, washed with water, extracted, dried, and distilled under reduced pressure to obtain 0.81 g of oily liquid compound (E)-2-(2,2-dimethylpropyleneamino)acetate methyl ester with a yield of 90%.
[0045] 1 H-NMR (500Hz, CDCl 3 ): 1 (9H, s), 3.66 (3H, s), 4.07 (2H, s), 7.46 (1H, s).
[0046] Two, 2-(2,2-dimethylpropyleneamino)-3-methyl ethyl glutarate (2)
[0047] Add 0.81g of compound 1 (0.517mmol) (prepared in step 1), 22ml of anhydrous THF into a 50ml flask, add 0.494g of lithium bromide (0.569mmol), and then add 0.589g of ethyl crotonate (0.517mmol, 0.64 ml), 0.786g 1,8-diazabicyclo(5,4,0)-7-undecene (0.517mmol, 0.77ml) was added, and the reaction was stirred at r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com