Synthesis method of new ligand for efficient catalysis of CuAAC reaction
A synthesis method and reaction technology, applied in the field of synthesis of functional structural molecules, can solve the problems of harsh reaction conditions, insufficient research and development requirements for ligands, difficult synthesis, etc., and achieve obvious effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]
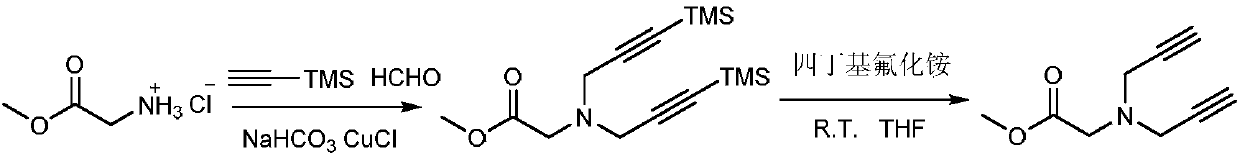
[0015] To synthesize glycine methyl ester TMS-diyne, add glycine methyl ester hydrochloride 2mmol trimethylsilyl acetylene 5mmol, mass concentration 5mmol of formaldehyde aqueous solution 5mmol, sodium bicarbonate 5mmol and cuprous chloride 0.2 in a round bottom flask mmol, mixed and stirred at 35°C for 18 hours, and the reaction process was monitored by TLC. After the reaction was completed, it was extracted three times with dichloromethane / water, and the organic phase was washed and dried, and the yield was 92%. The product is a colorless liquid. To synthesize glycine methyl ester diyne, add glycine methyl ester TMS-diyne 2mmol in 5mL tetrahydrofuran solution in a round bottom flask, then add tetrabutylammonium fluoride 5mmol in batches, mix and stir at room temperature for 3 hours, and monitor the reaction process by TLC . After the reaction was finished, it was extracted three times with dichloromethane / water, and the organic phase was washed and dried, and t...
Embodiment 2
[0017]
[0018]
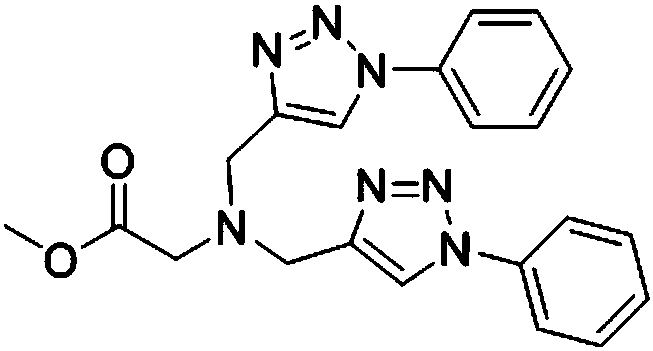
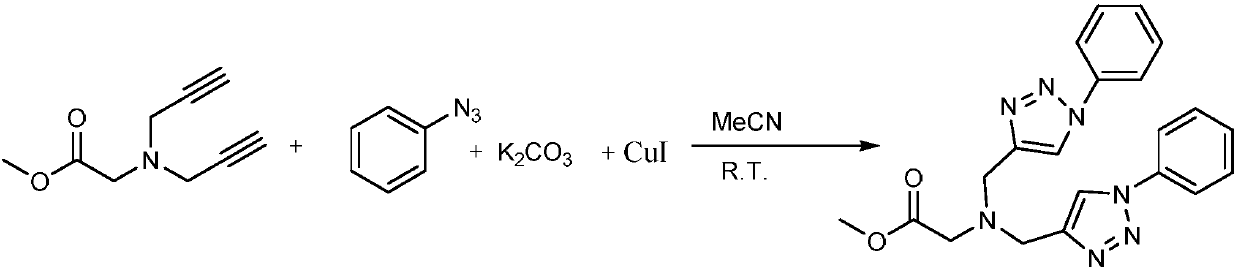
[0019] In a 10mL round bottom flask, add 1mmol glycine methyl ester diyne, 2mmol phenyl azide, 1.2mmol K 2 CO 3 and 0.1mmol cuprous iodide, mixed and stirred at room temperature in acetonitrile and water solvent for 10 hours, the reaction process was detected by TLC, extracted with ethyl acetate after the reaction, and separated by silica gel column chromatography to obtain the target product, the yield was 85% .
Embodiment 3
[0021]
[0022] In a 10mL round bottom flask, add 1mmol glycine methyl ester diyne, 2mmol p-trifluoromethylphenyl azide, 1.2mmol K 2 CO 3 and 0.1mmol cuprous iodide, mixed and stirred in acetonitrile and water solvent at room temperature for 12 hours, the reaction process was detected by TLC, extracted with ethyl acetate after the reaction, and separated by silica gel column chromatography to obtain the target product with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com