Preparation method of (S)-oxiracetam
A technology of glycine ethyl ester hydrochloride and hydroxyl, which is applied in the field of preparation-oxiracetam and preparation of oxiracetam, can solve the problems of increasing reaction steps, low synthesis yield, reduction of total yield and the like, and achieves reduction of impurities Content, low pollution, the effect of reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
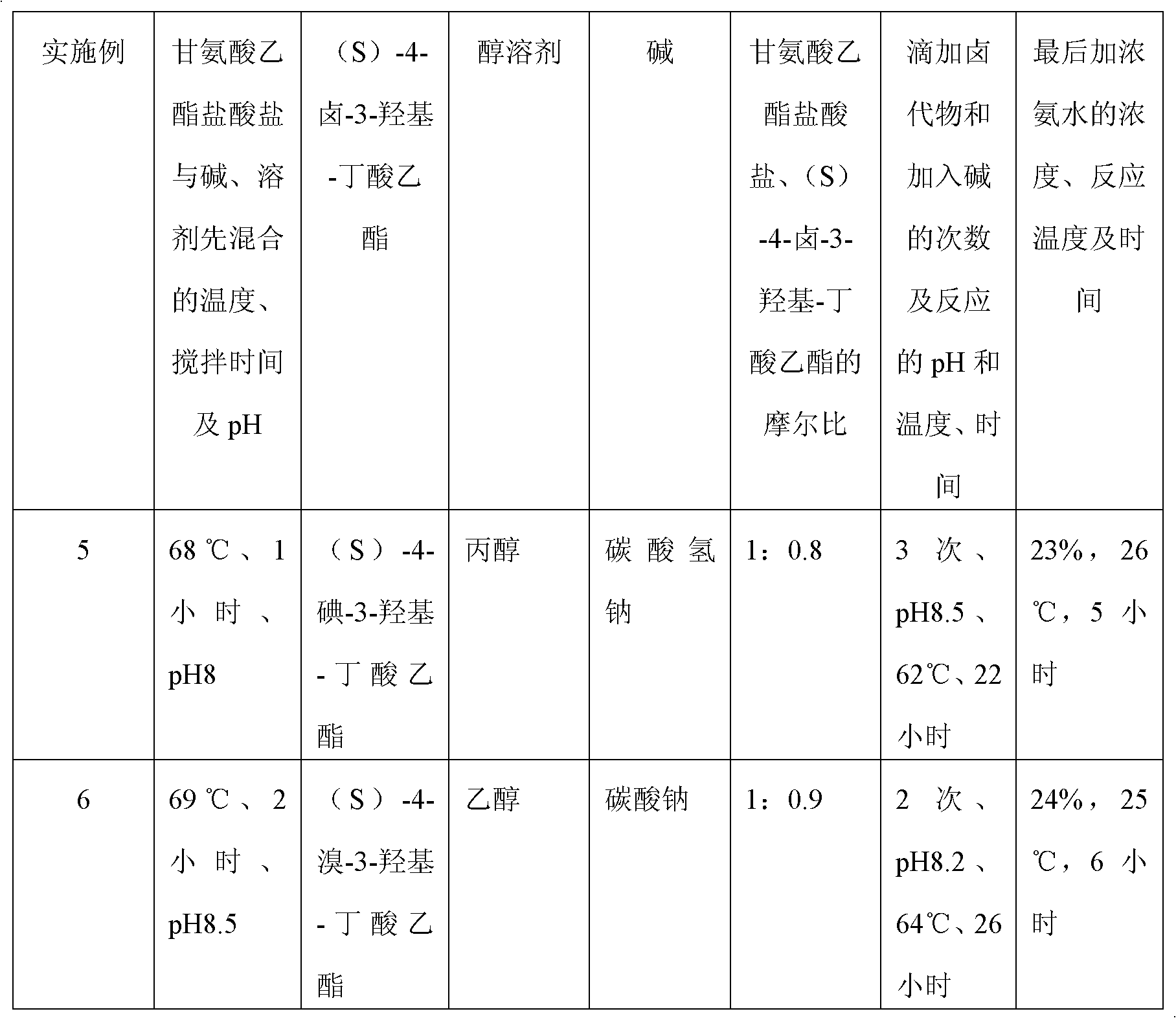
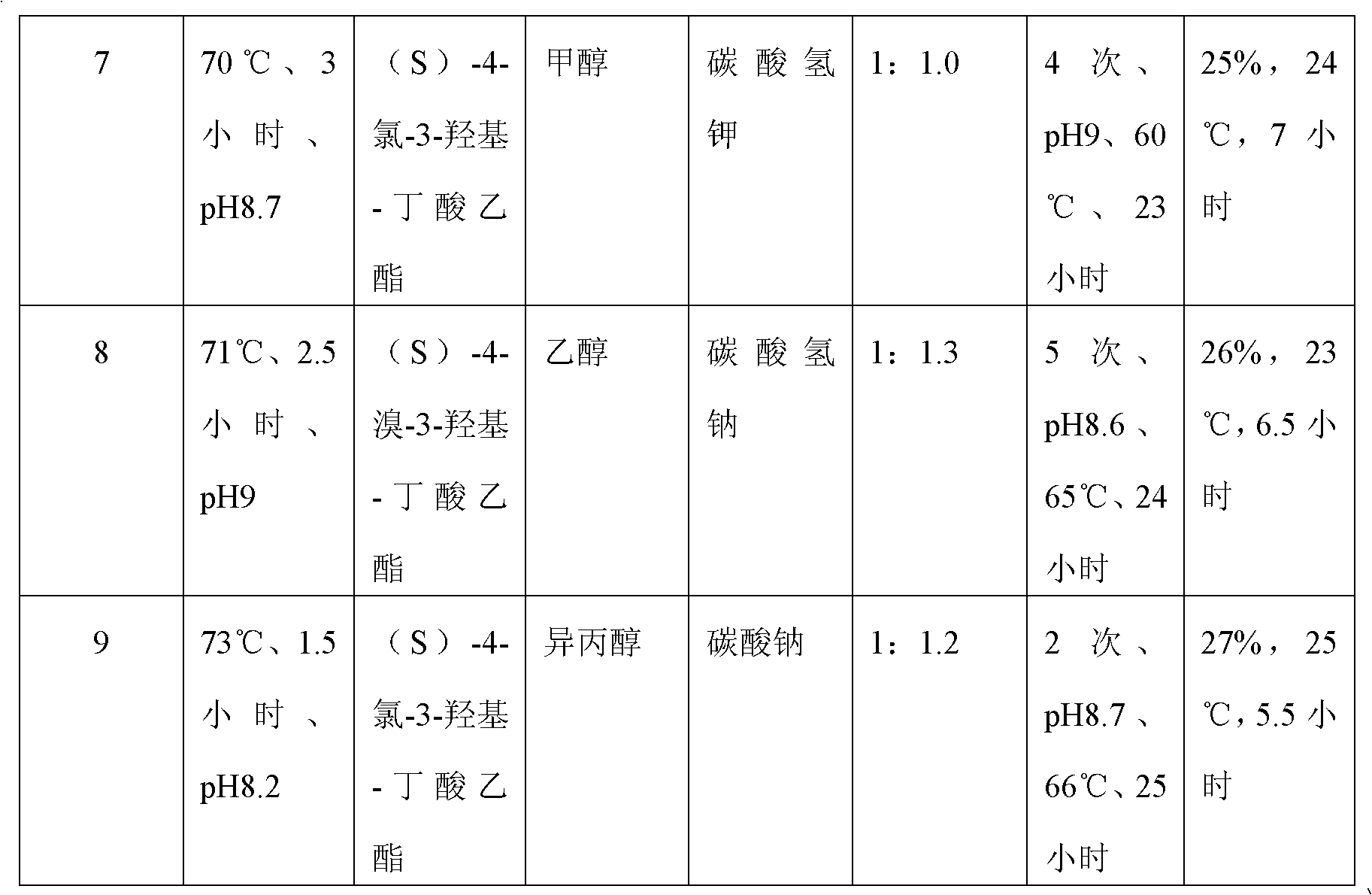
Embodiment 1
[0028] A kind of preparation method of (S)-oxiracetam, carry out as follows:
[0029] 1. Preparation of crude product:
[0030] (a) Mix 139.6g of glycine ethyl ester hydrochloride with 84.0g of sodium bicarbonate and 1008ml of absolute ethanol at 70°C and pH 8.7 for 2 hours, then add dropwise (S)-4-chloro-3-hydroxy - 183.3 g of ethyl butyrate, 84.0 g of sodium bicarbonate was added in two portions to control the pH of the reaction system to 9, and the reaction was carried out at 62 to 64°C for 24 to 25 hours. The dropwise addition of (S)-4-halogen-3 The time of -hydroxyl-butyric acid ethyl ester is 2.5 hours; Wherein the mol ratio of glycine ethyl ester hydrochloride and sodium bicarbonate is 1: 2, and dehydrated alcohol consumption is 6 times of alkali weight, glycine ethyl ester hydrochloride and The mol ratio of (S)-4-chloro-3-hydroxyl-butyric acid ethyl ester is 1: 1.1;
[0031] (b) then filter, fully wash the filtrate with inorganic alcohol, concentrate, the concentrate...
Embodiment 2
[0037] A kind of preparation method of (S)-oxiracetam, carry out as follows:
[0038] 1. Preparation of crude product:
[0039] (a) Mix glycine ethyl ester hydrochloride with alkali and alcohol solvent at 68-73°C and pH 8-9 for 1-3 hours, then add dropwise (S)-4-halo-3-hydroxy-butyl Acetate ethyl ester, adding alkali in stages to control the pH of the reaction system is 8-9 to fully react;
[0040] (b) then filter, fully wash the filtrate with inorganic alcohol, concentrate, and the concentrate is dissolved in water, then add 5 times the weight of the filtrate in dichloromethane to extract, concentrate the water phase; Crude Piracetam.
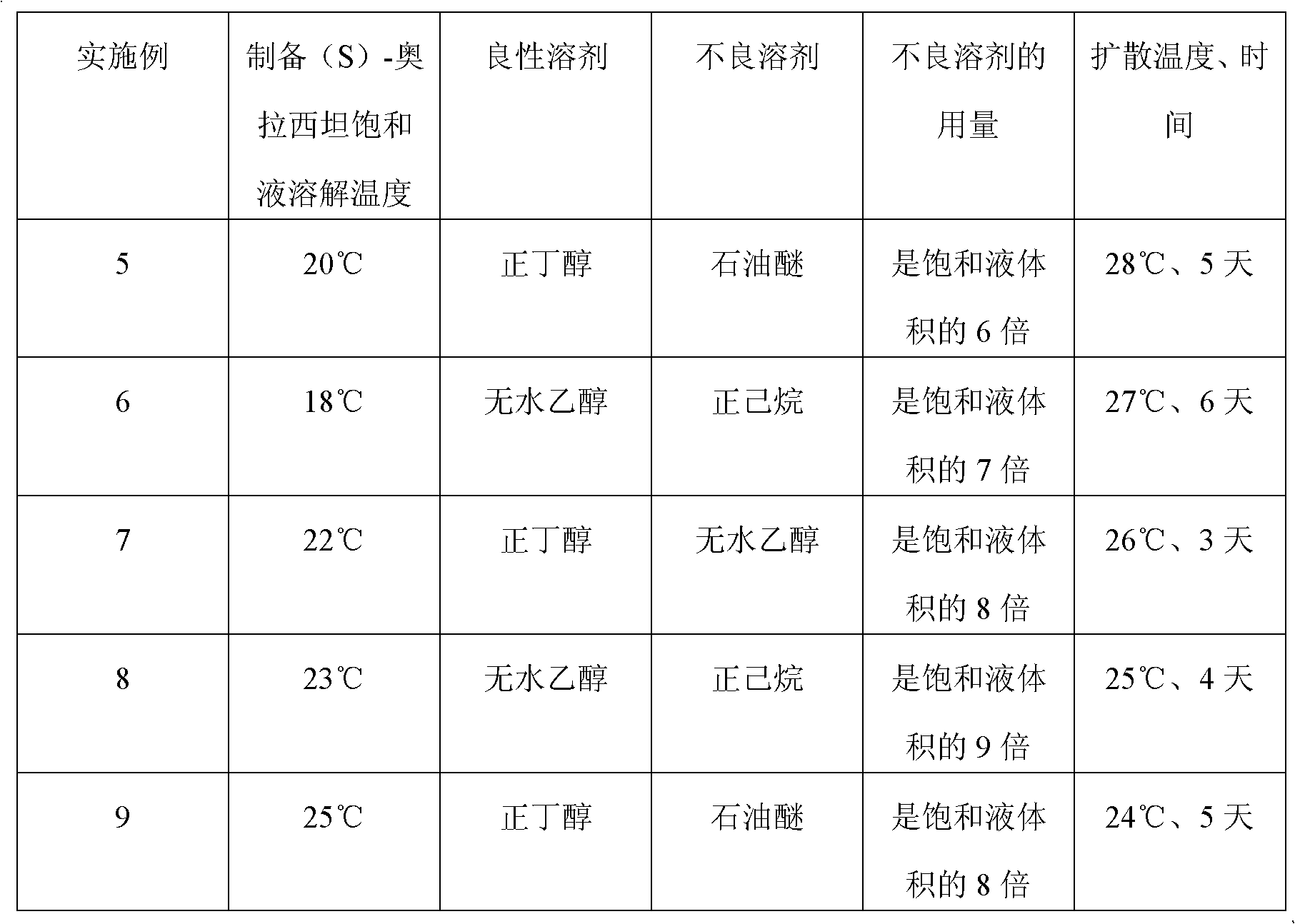
[0041] 2. Purification of crude product:
[0042] (a) dissolving the above-mentioned crude product in water, passing through 732# strongly acidic cation exchange resin, then neutralizing and collecting the solution, concentrating through 711# strongly basic anion exchange resin; described crude product: water=1 gram: 0.8 milliliters, Descr...
Embodiment 3
[0046] A kind of preparation method of (S)-oxiracetam, carry out as follows:
[0047] 1. Preparation of crude product:
[0048] (a) Mix glycine ethyl ester hydrochloride with sodium bicarbonate and anhydrous methanol at 68-73°C and pH 8 for 3 hours, then add dropwise (S)-4-halo-3-hydroxy-butyric acid Ethyl ester, sodium bicarbonate is added in portions to control the pH of the reaction system to be 8.8 for full reaction; wherein the molar ratio of glycine ethyl ester hydrochloride to sodium bicarbonate is 1:2, and the amount of dehydrated alcohol is 5-8 of the alkali weight times;
[0049] (b) Then filter, fully wash the filtrate with inorganic alcohol, concentrate, dissolve the concentrate in water, then add ethyl acetate for extraction, concentrate and separate the aqueous phase; finally add concentrated ammonia water to react to obtain (S)-oxiracetam Crude;
[0050] 2. Purification of crude product:
[0051] (a) Dissolving the crude product obtained above in water, pas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com