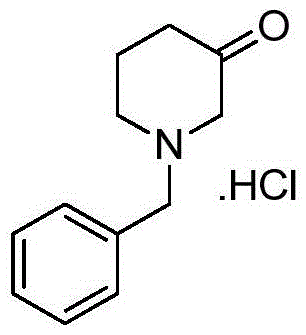
Preparation method for 1-benzyl-3-piperidone hydrochloride
A technology of piperidone hydrochloride and benzylglycine ethyl ester is applied in the field of preparation of 1-benzyl-3-piperidone hydrochloride, can solve the problem of high cost, achieve good quality, short synthesis steps, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
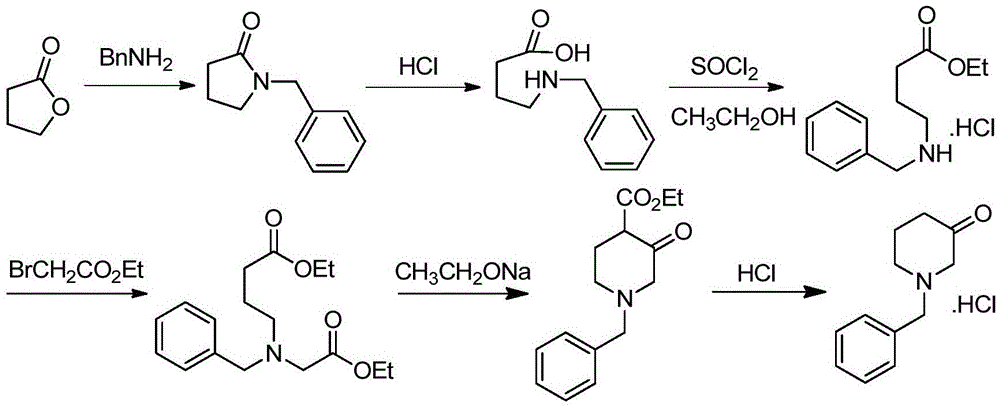
[0034] Embodiment 1 prepares 1-benzyl-3-piperidone hydrochloride
[0035] Follow the steps below:
[0036] (1) Preparation of Intermediate IV
[0037] 43g (0.4mol) of benzylamine, 250mL of acetonitrile, 13.3g (0.04mol) of tetrabutylammonium hydrogen sulfate, and 40.4g (0.4mol) of triethylamine were added successively to a 1000mL three-necked flask. A solution of 49g (0.4mol) of ethyl acetate and 150mL of acetonitrile, keep it warm at 25-28°C for 4h after dropping, HPLC detects that the reaction is complete, cool the reaction solution to 0-5°C and let it stand for 1.5h, filter, and depressurize the filtrate Concentrate to obtain 80.5 g (104% yield) of a light brown oily substance (ie Intermediate IV), with a purity of 98.2% as detected by HPLC, which is directly put into the next reaction.
[0038] (2) Preparation of Intermediate III
[0039] Add 80.5g (0.4mol) of intermediate IV prepared in the previous step to a 1000mL three-necked flask, add 500mL of tetrahydrofuran to di...
Embodiment 2
[0044] Embodiment 2 prepares 1-benzyl-3-piperidone hydrochloride
[0045] Follow the steps below:
[0046] (1) Preparation of Intermediate IV
[0047]43g (0.4mol) of benzylamine, 250mL of dichloromethane, 45.6g (0.2mol) of benzyltriethylammonium chloride, and 154.8g (1.2mol) of diisopropylethylamine were successively added to a 1000mL three-necked flask. Stir, add dropwise a solution of 217.3g (1.2mol) of ethyl bromoacetate and 150mL of dichloromethane, keep warm at 25-28°C for 4 hours after dropping, HPLC detects that the reaction is complete, and cool the reaction solution to 0-5°C Let stand for 1.5h, filter, and concentrate the filtrate under reduced pressure to obtain 75.5g (yield 97.7%) of a light brown oily substance (ie, intermediate IV), with a purity of 98.4% as detected by HPLC, which was directly put into the next reaction.
[0048] (2) Preparation of intermediate III
[0049] Add 75.5g (0.39mol) of intermediate IV prepared in the previous step to a 1000mL three-...
Embodiment 3
[0054] Embodiment 3 prepares 1-benzyl-3-piperidone hydrochloride
[0055] Follow the steps below:
[0056] (1) Preparation of Intermediate IV
[0057] 43g (0.4mol) of benzylamine, 250mL of toluene, 15g (0.057mol) of dodecyltrimethylammonium chloride, and 39.5g (0.5mol) of pyridine were successively added to a 1000mL three-necked flask. A solution of 55 g (0.45 mol) of ethyl chloroacetate and 150 mL of toluene, after dropping, keep it warm at 25-28 ° C for 4 h, after the reaction is detected by HPLC, the reaction solution is cooled to 0-5 ° C and allowed to stand for 1.5 h, filtered, and the filtrate Concentrated under reduced pressure to obtain 81.0 g (yield 105%) of a light brown oily substance (ie Intermediate IV), with a purity of 96.2% as detected by HPLC, which was directly put into the next reaction.
[0058] (2) Preparation of intermediate III
[0059] Add 1.0g (0.42mol) of the intermediate IV prepared in the previous step to a 1000mL three-necked flask, add 500mL of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com