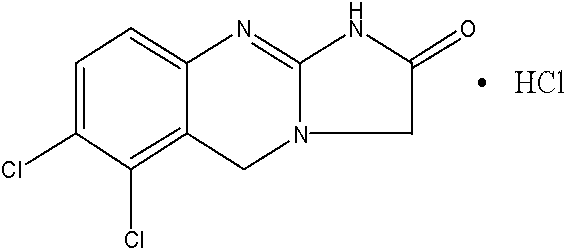
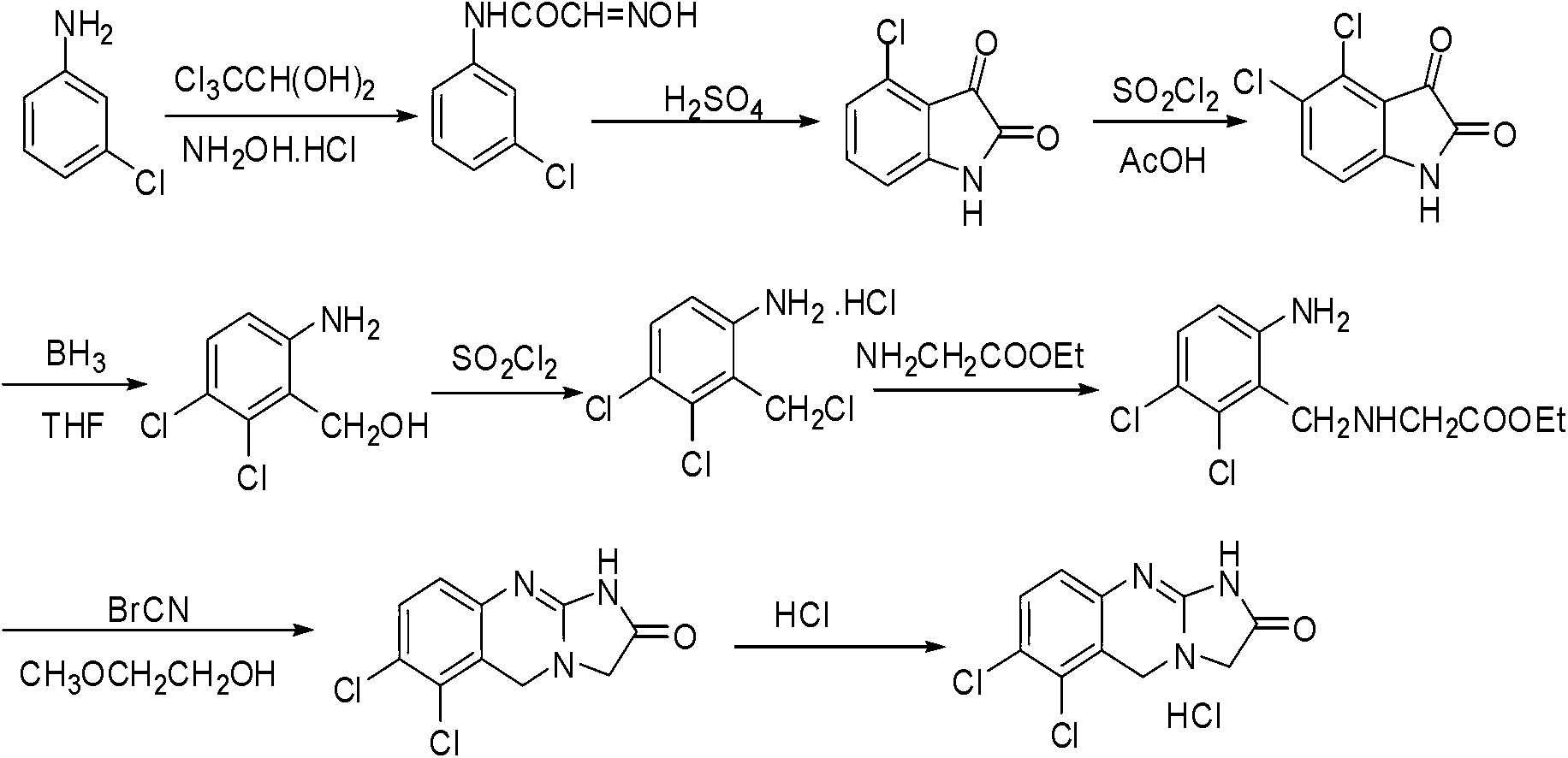
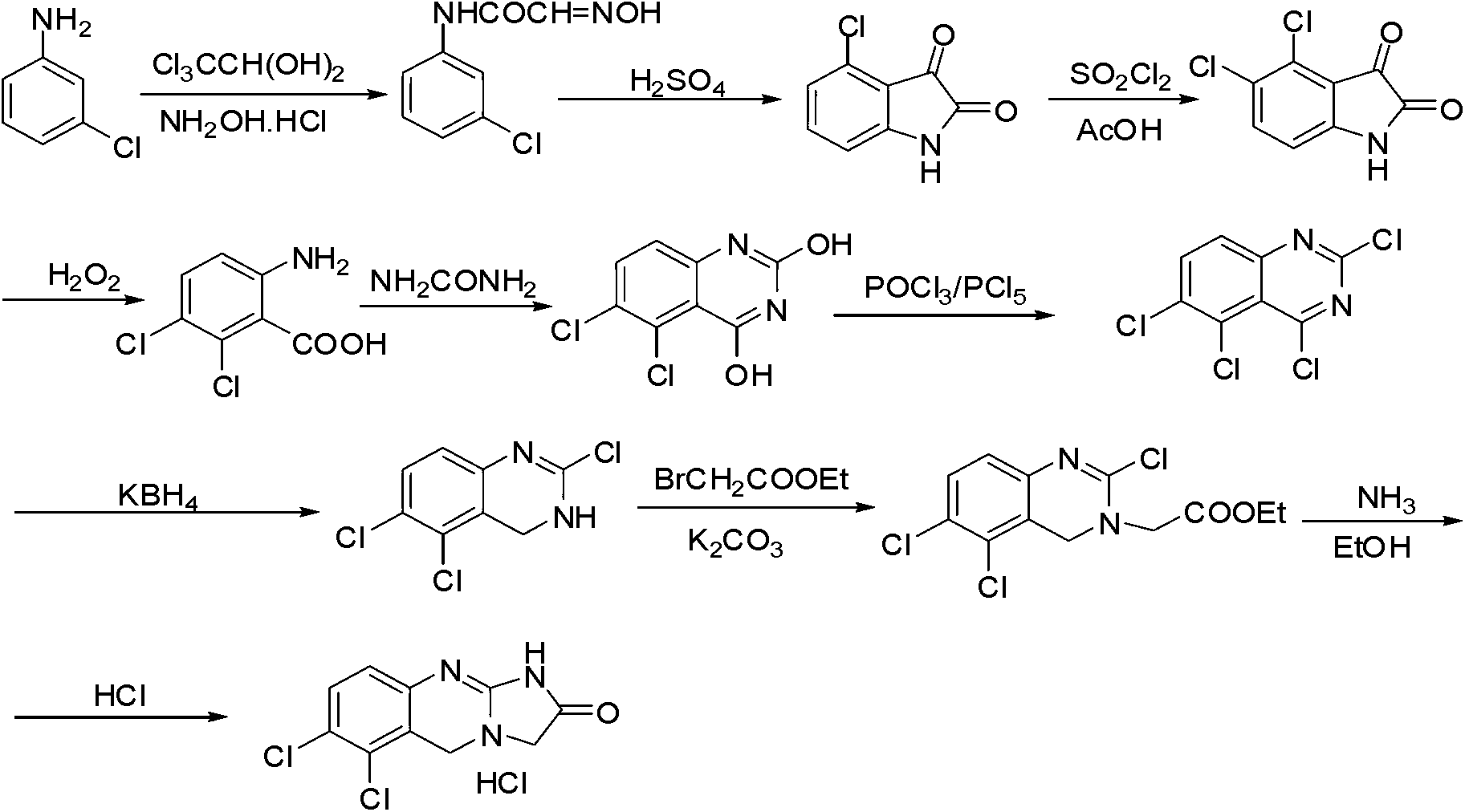
Preparation method of anagrelide hydrochloride
A technology of anagrelide hydrochloride and Lewis acid, which is applied in the direction of organic chemistry and the like, can solve the problems of long synthesis route of anagrelide hydrochloride, difficult product purification, high cost per unit consumption, and achieves less impurities, short synthesis route and single cost. low consumption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0030] Specific embodiment one: the preparation method of a kind of anagrelide hydrochloride of the present embodiment is carried out according to the following steps:
[0031] 1. Add 2000mL of THF into the three-necked flask, under the protection of nitrogen, add sodium borohydride, Lewis acid and 2,3-dichlorobenzoic acid, stir well at room temperature, add 260-270g of iodine in THF solution dropwise, drop After the addition, heat to reflux for 12-24 hours, then cool to room temperature, add methanol dropwise until the solution is clear, concentrate under reduced pressure, add 2000 mL of KOH solution with a mass concentration of 20%, hydrolyze at 20-60°C for 5-10 hours, and then Extract with a mixed solvent of dichloromethane / ethyl acetate, combine the organic layers, dry, filter, concentrate, and recrystallize from toluene to obtain 2,3-dichlorobenzyl alcohol as a white solid; wherein, 2,3-dichlorobenzoic acid, The mol ratio of sodium borohydride and Lewis acid is 1: (2~3): ...
specific Embodiment approach 2
[0043] Embodiment 2: This embodiment is different from Embodiment 1 in that: in step 1, hydrolyze at 40° C. for 5 hours. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0044] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the Lewis acid in step 1 is aluminum trichloride, tin dichloride, zinc chloride, ferric chloride or boron trifluoride. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com