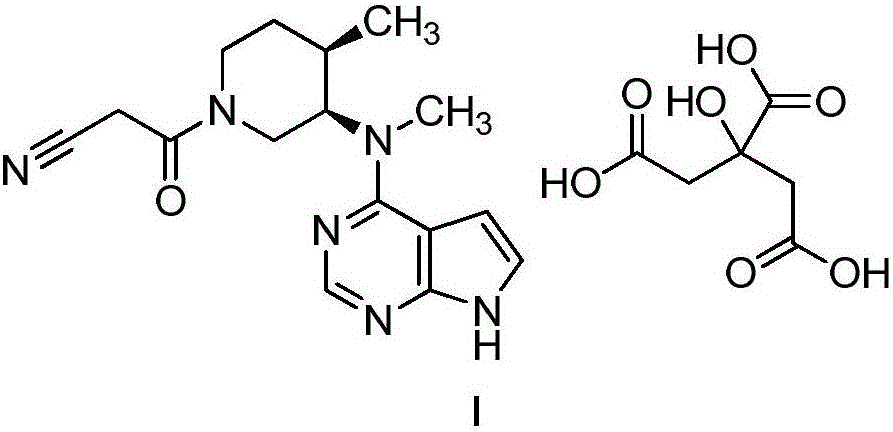
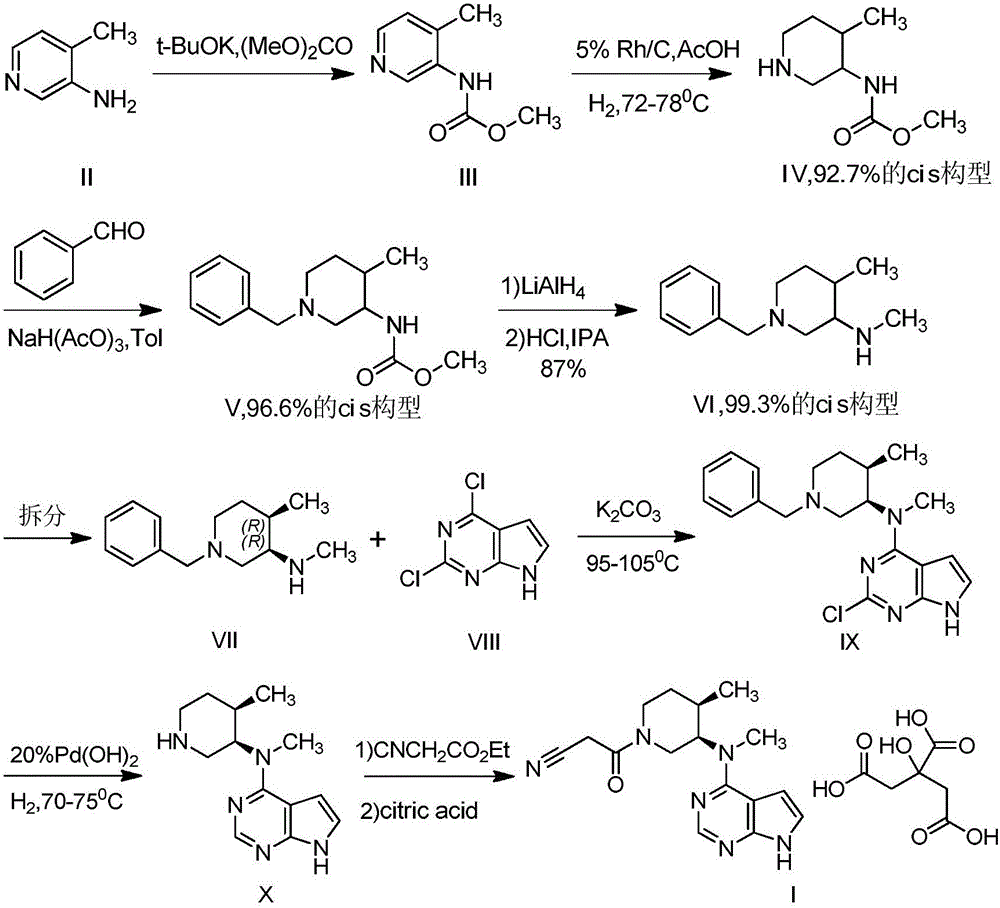
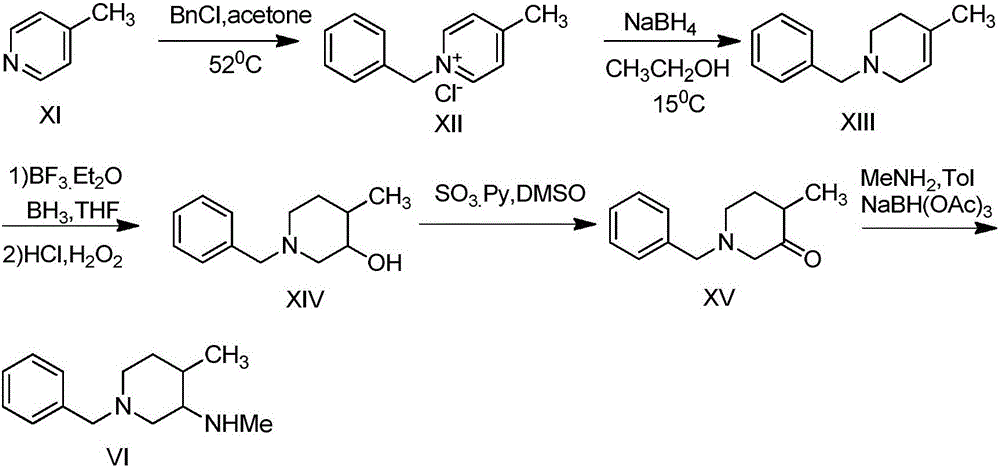
Synthesis method of N-benzyl-4-methylpiperidine-3-one hydrochloride
The technology of a kind of methylpiperidine and synthesis method is applied in the field of synthesis of N-benzyl-4-methylpiperidin-3-one hydrochloride, which can solve the problems of high production cost and achieve good product quality and simple synthesis steps. The effect of short, simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Add 96.5g (0.5mol) of N-benzylglycine ethyl ester and 123.5g (0.75mol) of ethyl 2-methyl-4-chlorobutyrate into a 2000mL reaction flask, add 650mL of carbon tetrachloride and triethylamine 76g (0.75mol), heated to reflux, reacted for 18h, after the detection of complete reaction, the reaction solution was cooled to 22 ° C, filtered, and the filtrate was concentrated under reduced pressure to obtain 165g of brown oil intermediate XVIII, the purity of which was detected by HPLC was 94.1% , directly into the next reaction.
[0032] (2) Add 165g of the concentrate intermediate XVIII prepared in the previous step to a 2000mL reaction flask, add 850mL of toluene to dissolve, add 96.1g (1.0mol) of sodium tert-butoxide, heat to reflux, and react for 5h. After the reaction of the raw materials is basically complete after HPLC detection , the reaction solution was cooled to room temperature, 55g of acetic acid was added dropwise, and the pH was adjusted to 6-8, 300mL of satura...
Embodiment 2
[0035] (1) Add 96.5g (0.5mol) of ethyl N-benzylglycine and 136g (0.65mol) of ethyl 2-methyl-4-bromobutyrate into a 2000mL reaction flask, add 850mL of tetrahydrofuran and 69g of sodium carbonate (0.65mol), heated to reflux, reacted for 22h, after detecting that the reaction was complete, the reaction solution was cooled to 22°C, filtered, and the filtrate was concentrated under reduced pressure to obtain 164.6g of brown oil intermediate XVIII, the purity of which was detected by HPLC was 94.8%. One step reaction.
[0036] (2) Add 164.6g of the brown oil prepared in the previous step to a 2000mL reaction flask, add 850ml of xylene, add 90.0g (0.8mol) of potassium tert-butoxide, heat to reflux, and react for 4.5h. The reaction of the raw materials is basically complete as detected by HPLC. Afterwards, the reaction solution was cooled to room temperature, 44 g of acetic acid was added dropwise to adjust the pH to 6-8, 280 mL of saturated saline was added, the layers were stirred ...
Embodiment 3
[0039](1) Add 96.5g (0.5mol) of ethyl N-benzylglycine and 136g (0.65mol) of ethyl 2-methyl-4-bromobutyrate into a 2000mL reaction flask, add 750mL of toluene and N,N - 76g (0.75mol) of diisopropylethylamine, heated to reflux, and reacted for 8h. After the reaction was detected to be complete, the reaction liquid was cooled to 24°C and filtered. The purity of the filtrate was detected by HPLC to be 93.3%, and the filtrate was directly put into the next reaction.
[0040] (2) Add the toluene filtrate prepared in the previous step into a 2000mL reaction flask, add 65.0g (0.95mol) of sodium ethoxide, heat to reflux, and react for 6.5h. 55g of acetic acid, adjust the pH to 6-8, add 400mL of tap water, stir to separate layers, transfer to a separatory funnel, wash the organic layer with saturated brine 300mL×2, then add anhydrous sodium sulfate to dry, filter, and concentrate the filtrate to obtain intermediate XIX 122.6 g (0.445 mol weight) of the oily matter, the HPLC detection pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com