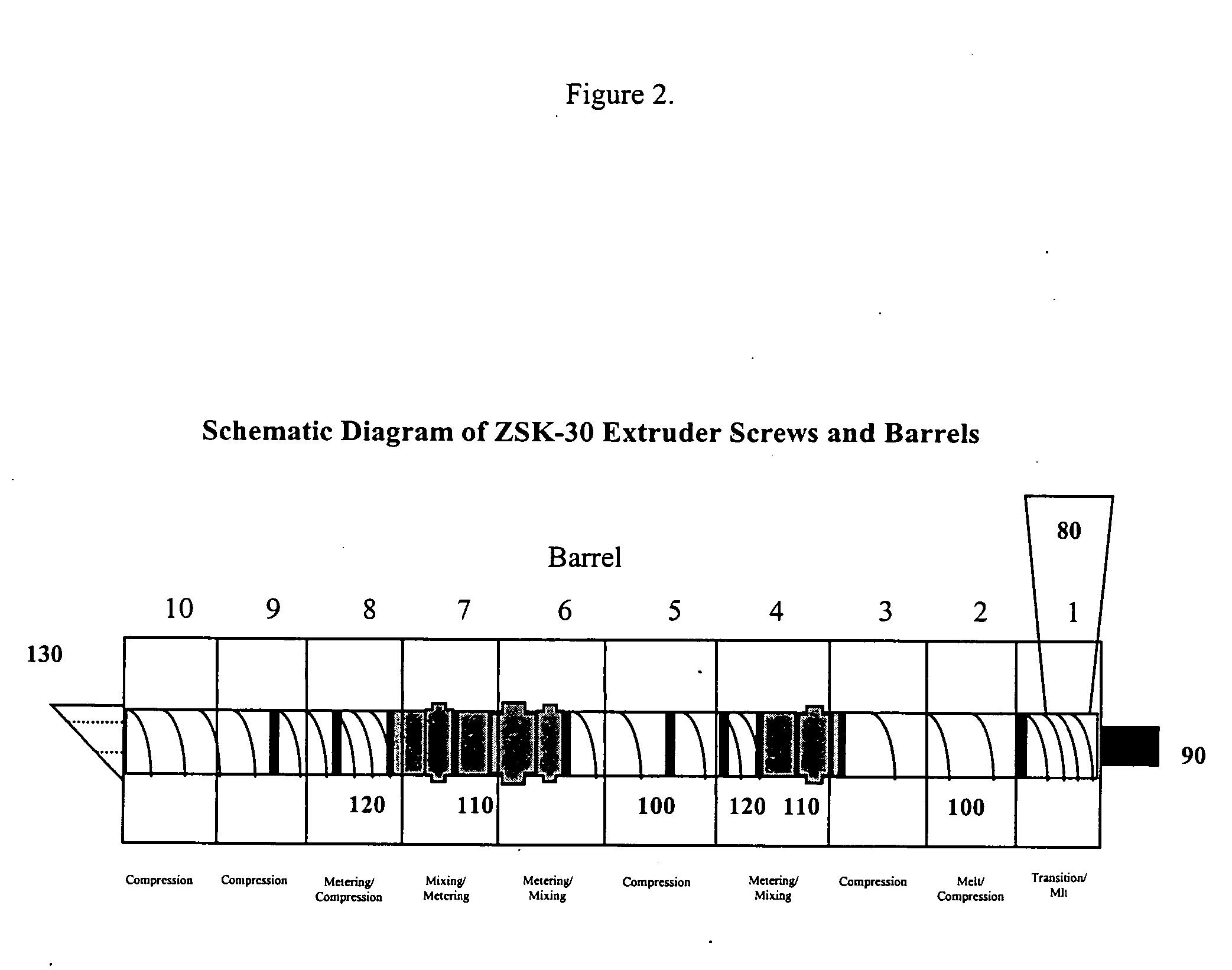
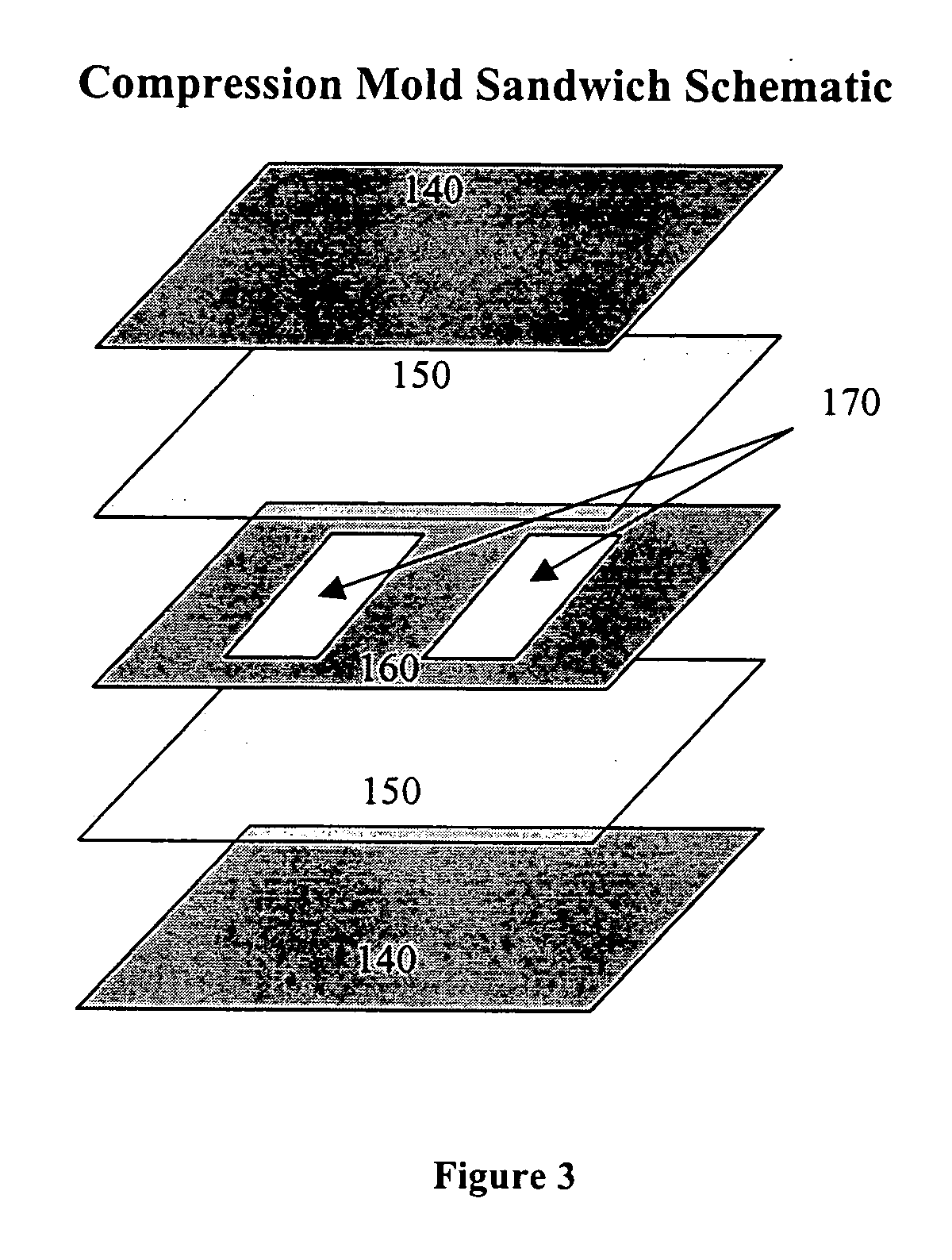
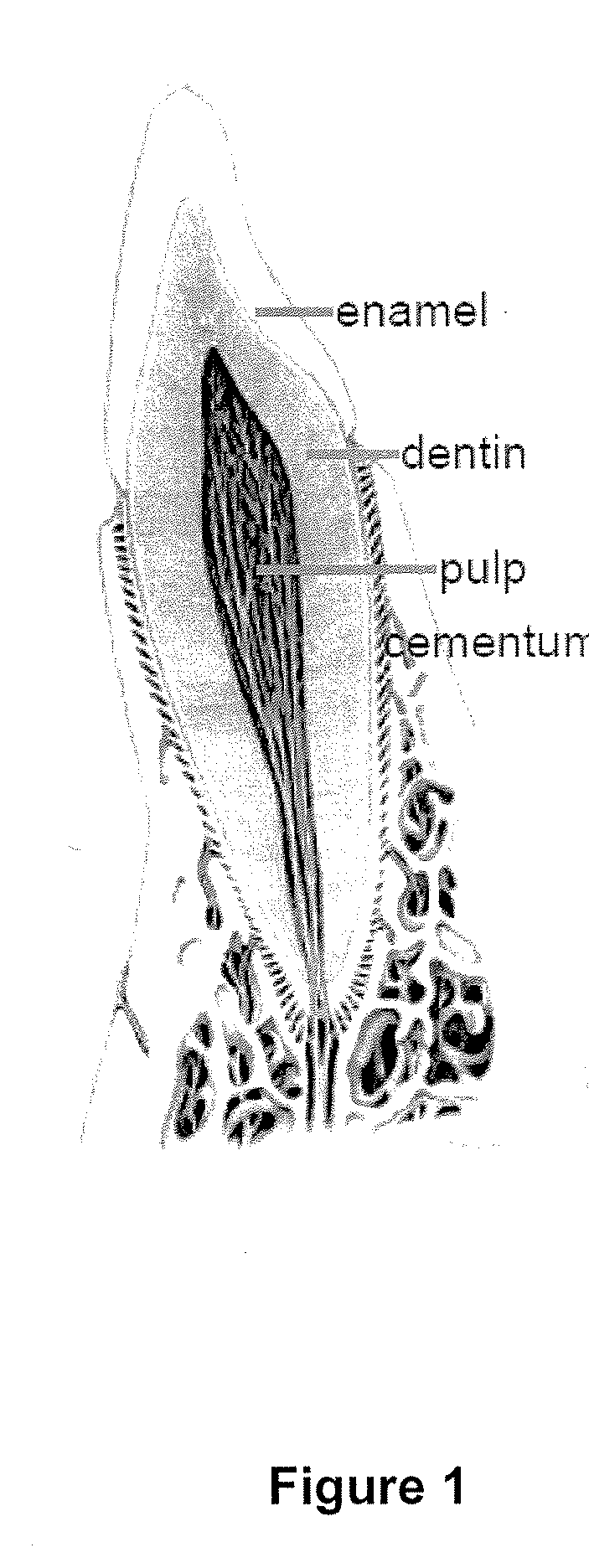
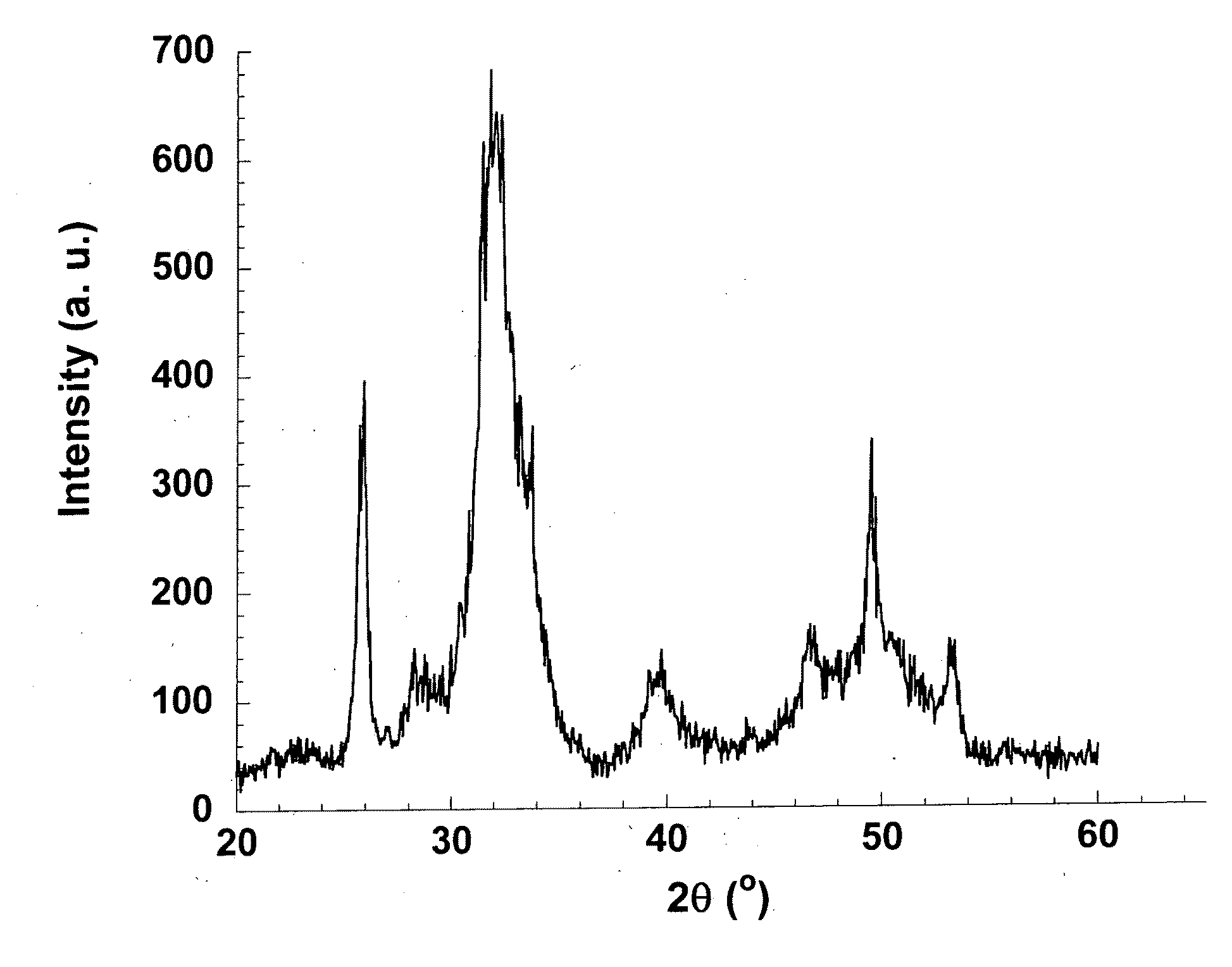
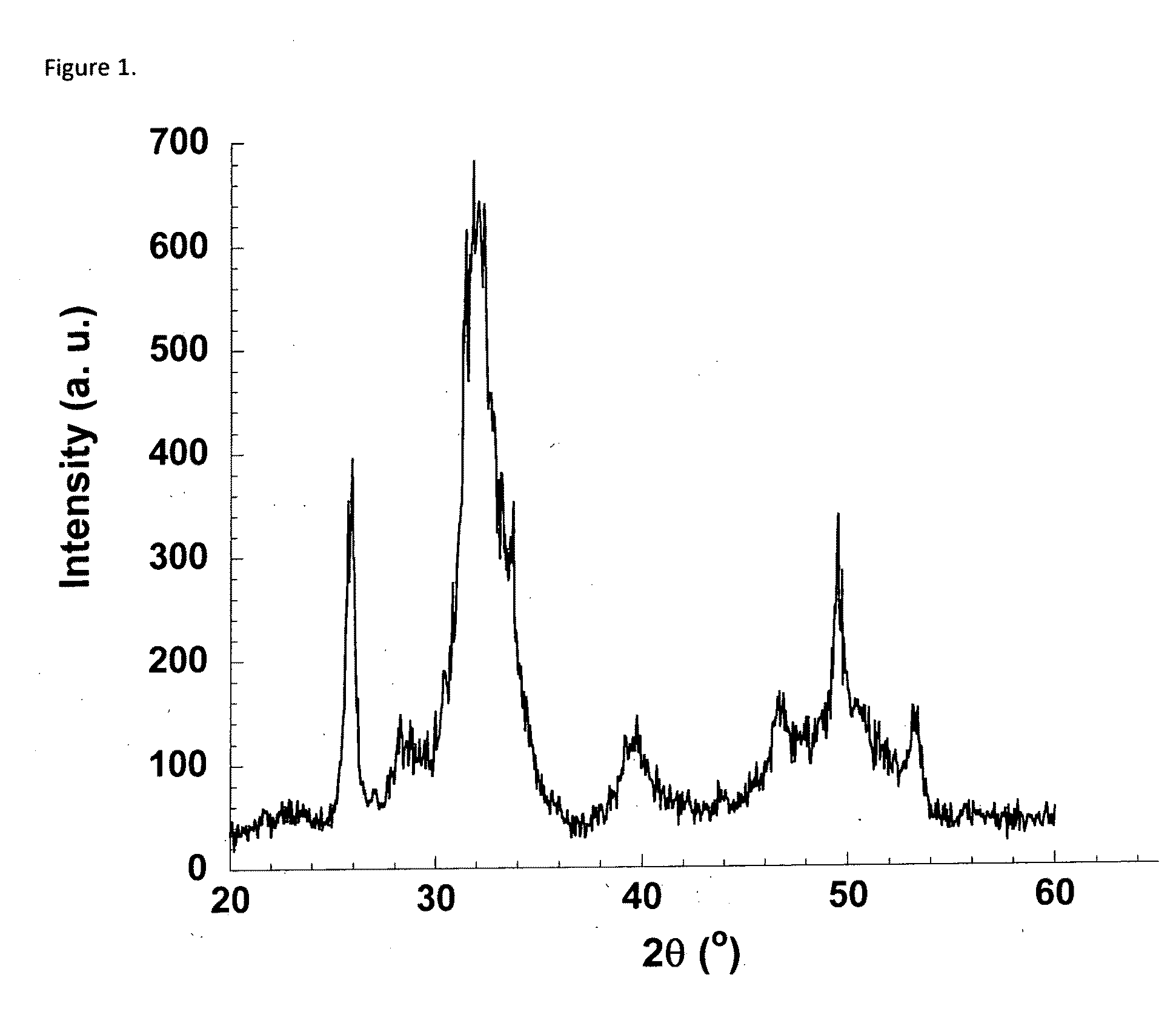
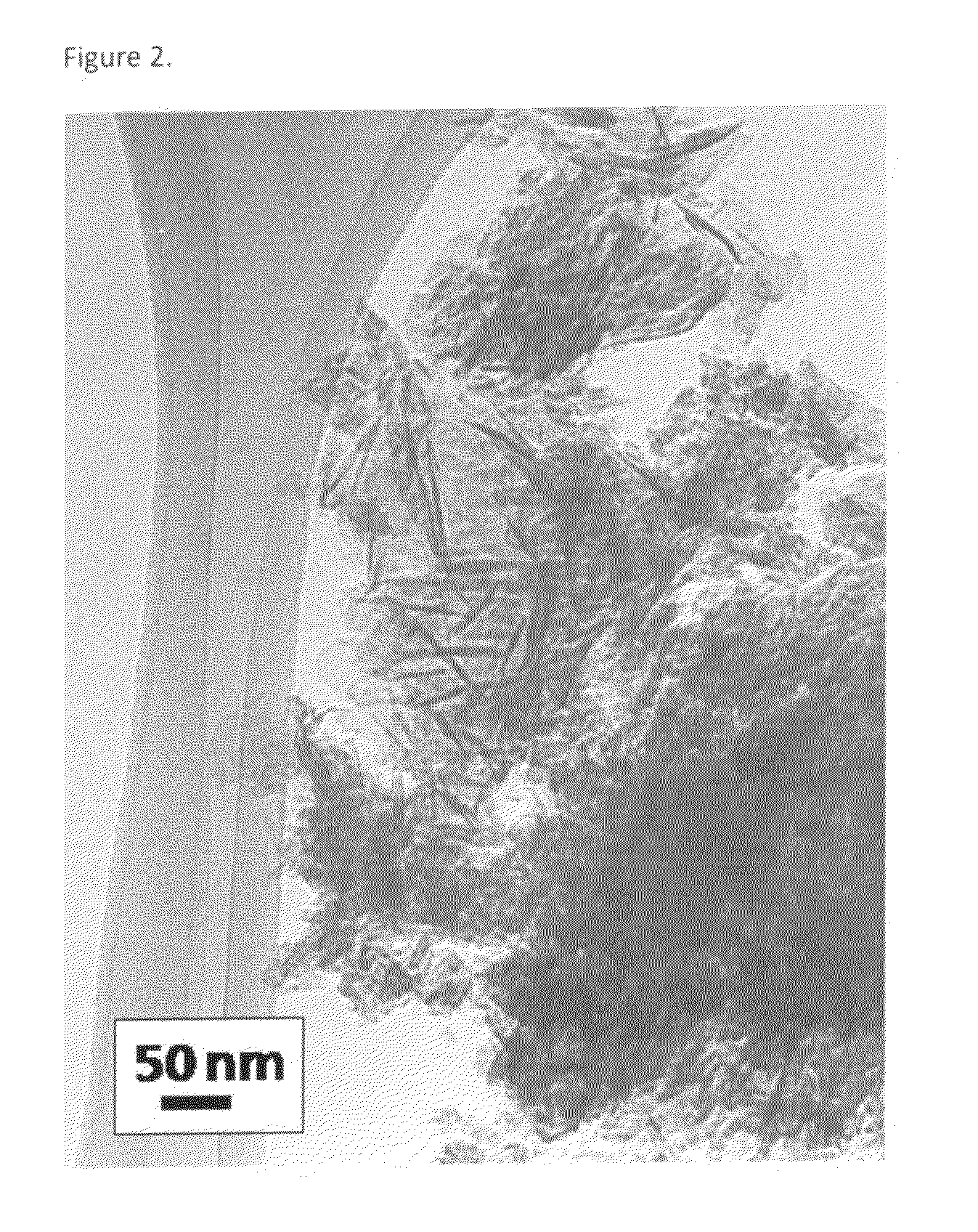
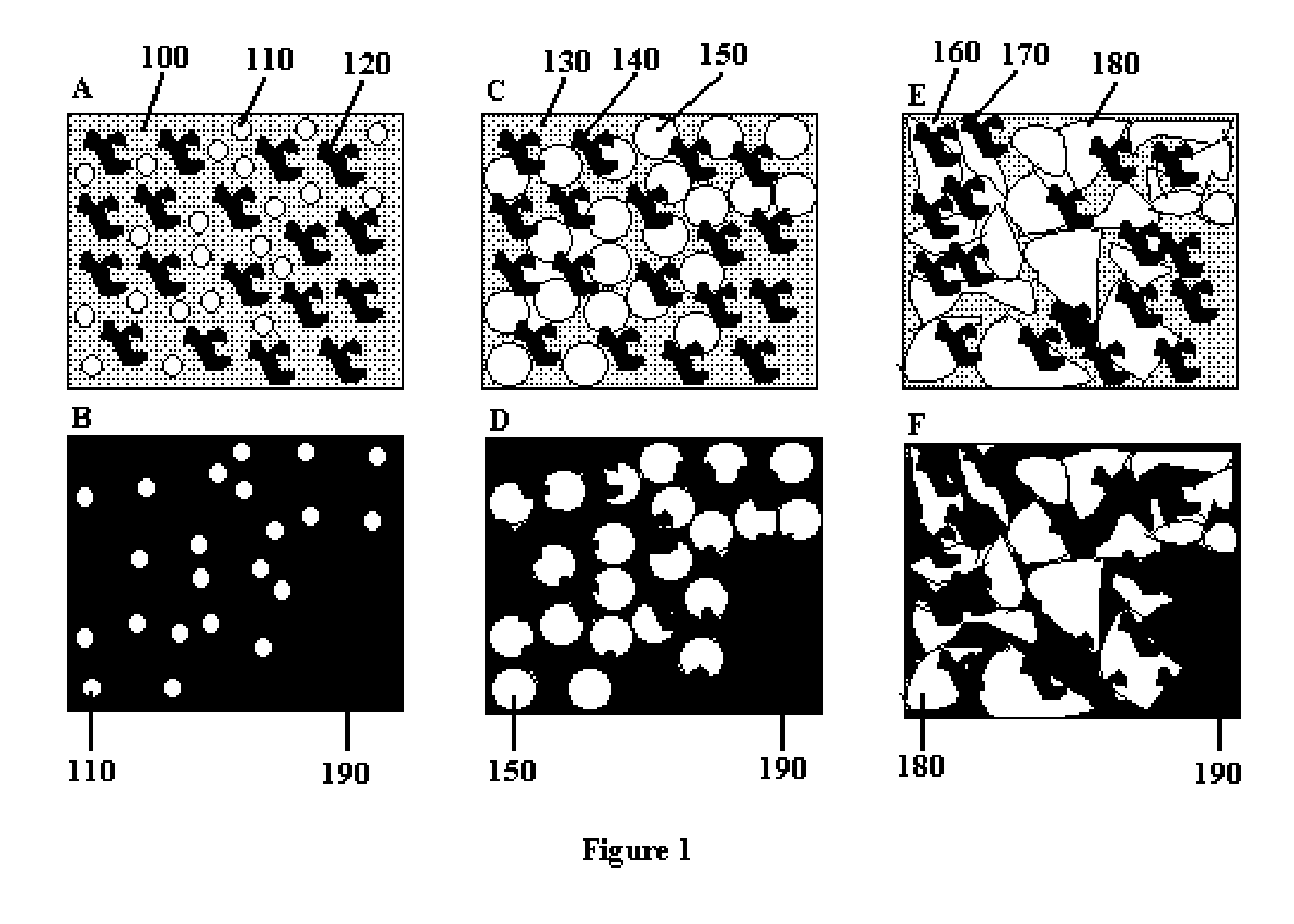
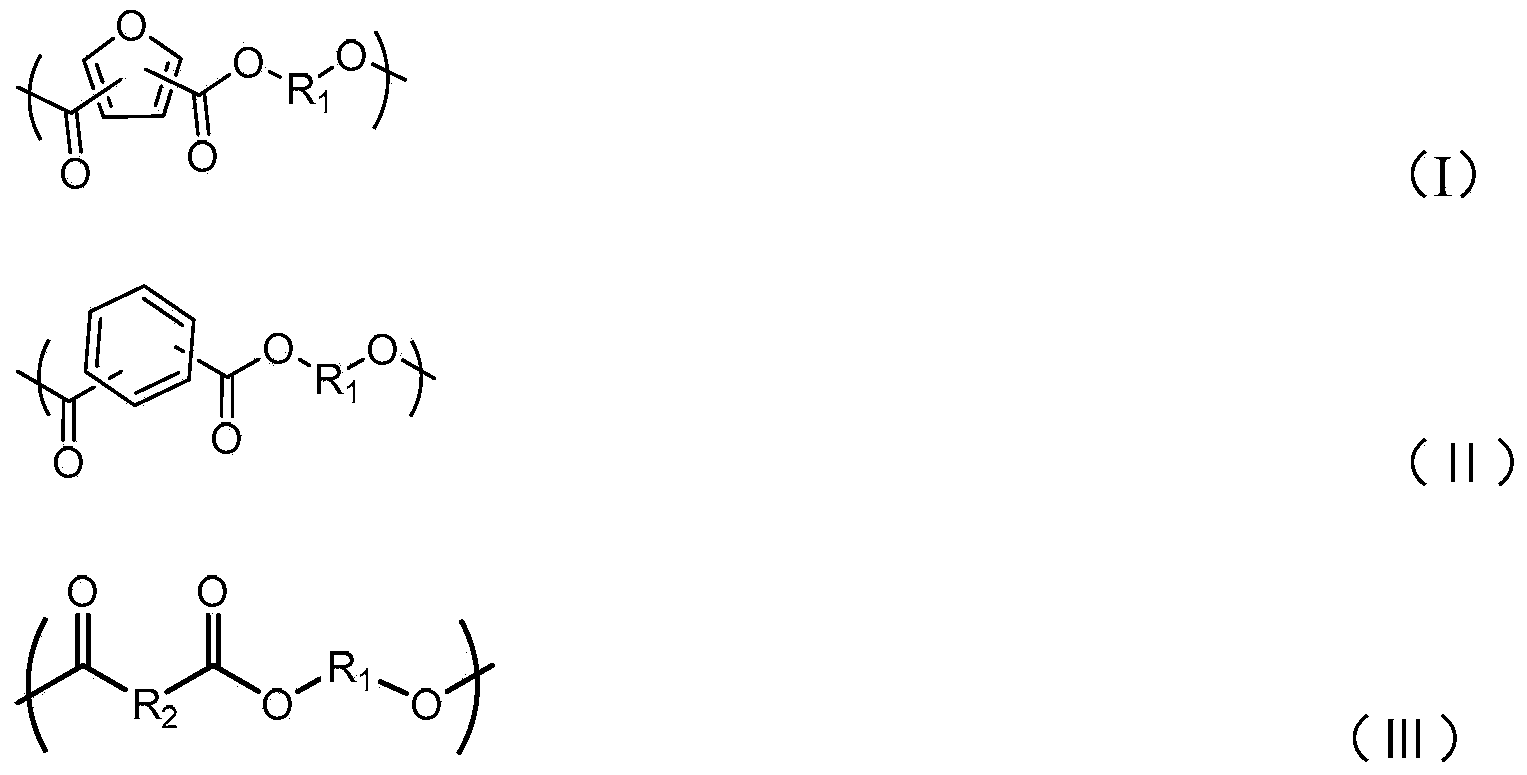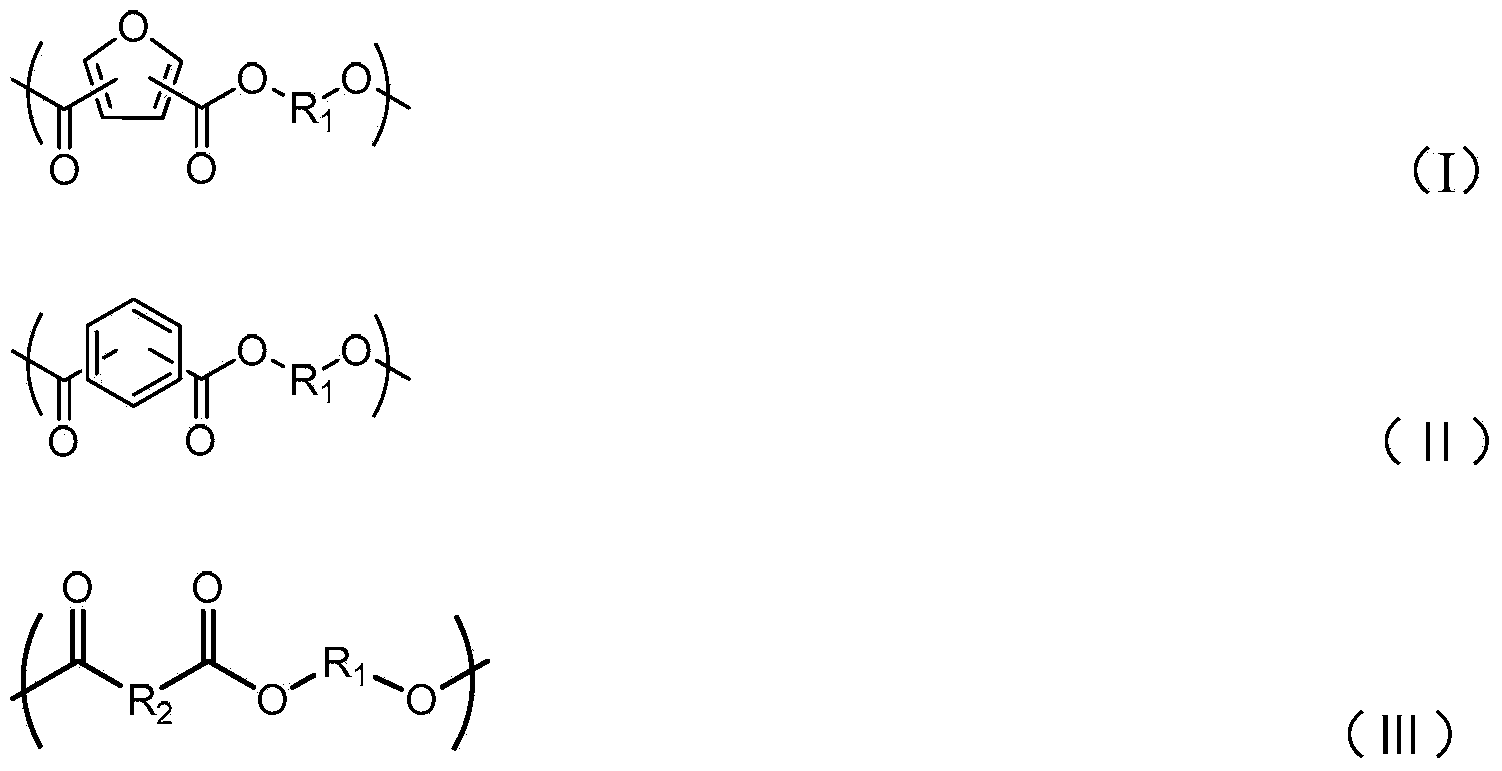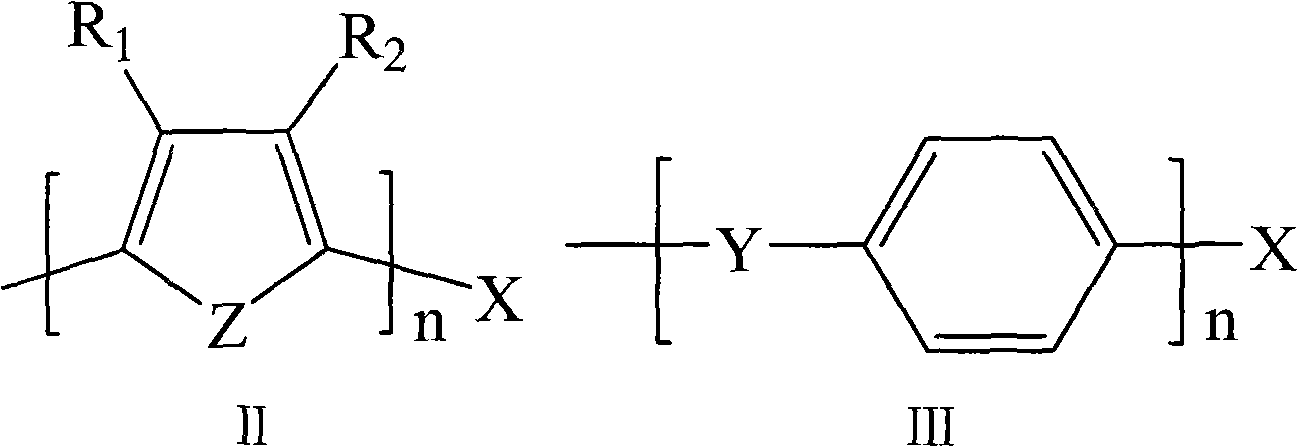Patents
Literature
218 results about "Polyglycolide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polyglycolide or poly(glycolic acid) (PGA), also spelled as polyglycolic acid, is a biodegradable, thermoplastic polymer and the simplest linear, aliphatic polyester. It can be prepared starting from glycolic acid by means of polycondensation or ring-opening polymerization. PGA has been known since 1954 as a tough fiber-forming polymer. Owing to its hydrolytic instability, however, its use has initially been limited. Currently polyglycolide and its copolymers (poly(lactic-co-glycolic acid) with lactic acid, poly(glycolide-co-caprolactone) with ε-caprolactone and poly (glycolide-co-trimethylene carbonate) with trimethylene carbonate) are widely used as a material for the synthesis of absorbable sutures and are being evaluated in the biomedical field.
Wound healing polymer compositions and methods for use thereof
The present invention provides bioactive polymer compositions that can be formulated to release a wound healing agent at a controlled rate by adjusting the various components of the composition. The composition can be used in an external wound dressing, as a polymer implant for delivery of the wound healing agent to an internal body site, or as a coating on the surface of an implantable surgical device to deliver wound healing agents that are covalently attached to a biocompatible, biodegradable polymer and / or embedded within a hydrogel. Methods of using the invention bioactive polymer compositions to promote natural healing of wounds, especially chronic wounds, are also provided. Examples of biodegradable copolymer polyesters useful in forming the blood-compatible, hydrophilic layer or coating include copolyester amides, copolyester urethanes, glycolide-lactide copolymers, glycolide-caprolactone copolymers, poly-3-hydroxy butyrate-valerate copolymers, and copolymers of the cyclic diester monomer, 3-(S)[(alkyloxycarbonyl)methyl]-1,4-dioxane-2,5-dione, with L-lactide. The glycolide-lactide copolymers include poly(glycolide-L-lactide) copolymers formed utilizing a monomer mole ratio of glycolic acid to L-lactic acid ranging from 5:95 to 95:5 and preferably a monomer mole ratio of glycolic acid to L-lactic acid ranging from 45:65 to 95:5. The glycolide-caprolactone copolymers include glycolide and ε-caprolactone block copolymer, e.g., Monocryl or Poliglecaprone.
Owner:MEDIVAS LLC
Environmentally friendly polylactide-based composite formulations
Polymeric materials and products, including sheet flooring materials prepared from the polymeric materials, and processes for preparing the polymeric materials, are disclosed. The polymeric materials include a polylactic acid-based polymer in combination with plasticizer and a compatibilizer, and optionally include a filler. The polymeric material can include between about 30 to about 50 percent by weight polyvinyl chloride, polyethylene glycol, polyglycolide, ethylene vinyl acetate, polycarbonate, polycaprolactone, polyhydroxyalkanoates, or polyolefins modified with polar groups, for example, ionomers. The plasticizer is typically an epoxidized vegetable oil or esterified and epoxidized vegetable oil and is typically present in an amount of between about 10 and about 50% by weight. In some embodiments, the compatibilizer is a polyolefin modified with one or more polar functional groups, and is typically present in an amount of between about 5 and about 10% by weight. The material can be used in decorative surface coverings, such as a floor coverings, particularly when it is in the form of a polymeric sheet. The polymeric material can be present in at least one layer of a floor covering, which floor covering can also include one or more additional layers such as wear layers, foamed or foamable layers, top coat layers and design layers. The additional layers can also include the polymeric material.
Owner:AFI LICENSING
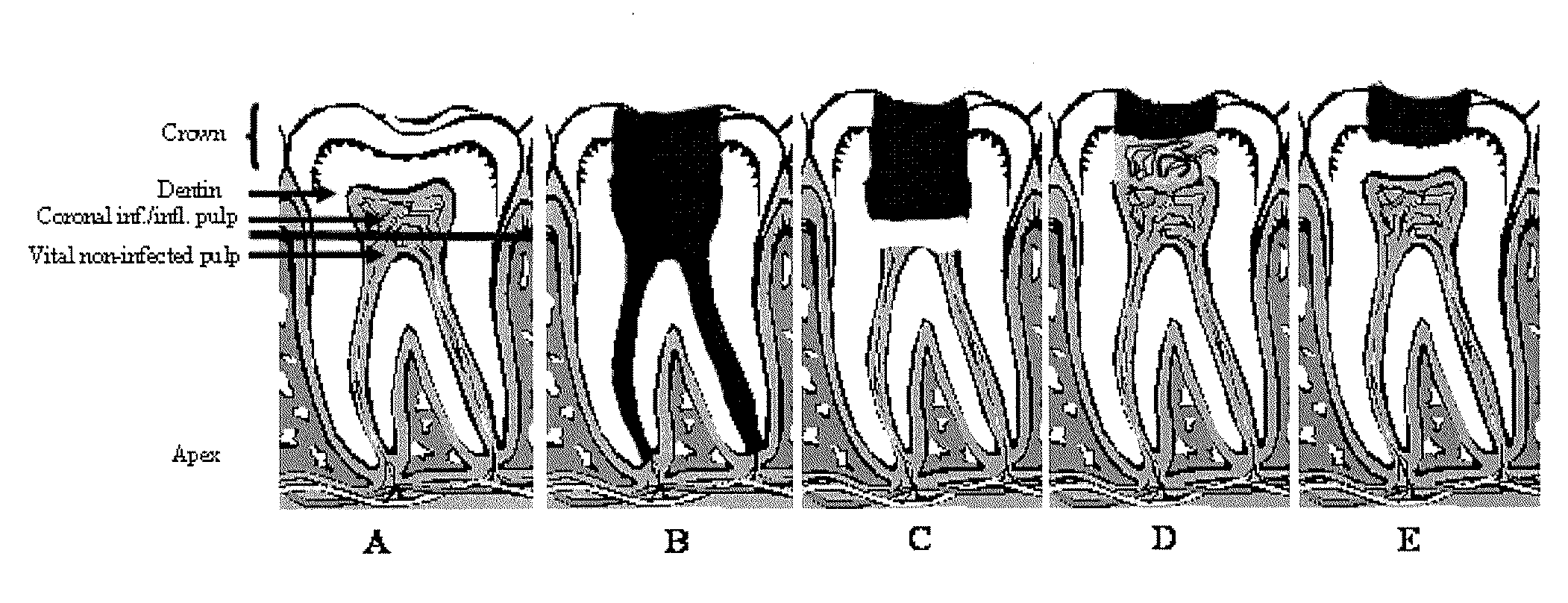
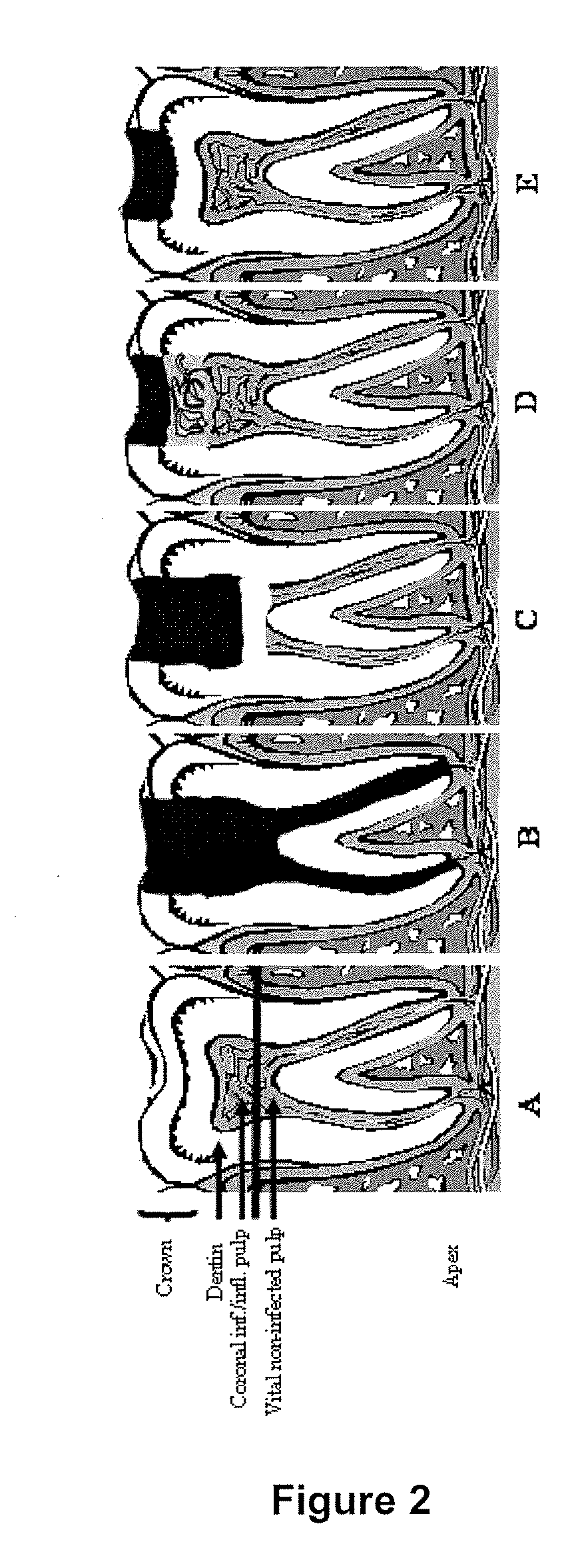
Compositions and methods for treating pulp inflammations caused by infection or trauma
The present disclosed subject matter relates to methods and compositions for restoring a diseased or damaged tooth such that infection is inhibited or eliminated and pulp regeneration is facilitated. The disclosed subject matter also includes a composition comprising a physiologically acceptable matrix seeded with pulp cells. The matrix can be capable of being injected into the pulp chamber of a tooth. In some embodiments, the matrix of a composition includes a hydrogel (e.g., collagen, chitosan, alginate, MATRIGEL™, gelatin, JELL-O®, fibrin), a mesh (e.g., polylactide-coglycolide (PLGA) mesh, polylactide (PLA) mesh, or polyglycolide (PGA) mesh, a cross-linked fiber mesh, a nanofiber mesh, a mesh fabric, biodegradable polymer mesh), a microsphere (biodegradable polymer microsphere, a hydrogel microsphere), or a combination of any of the foregoing. In yet other embodiments, the matrix includes a nanofiber, an artificial three-dimensional scaffold material, or a synthetic three-dimensional scaffold material.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20050065214A1Stimulate bone healing processLower potentialBiocidePowder deliveryBarium saltTG - Triglyceride
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20060013857A1Lower potentialMinimally inhibit osteogenesis and subsequent bone healingSurgical adhesivesSkeletal disorderBarium saltApatite
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters unsubstituted and N-substituted pyrrolidones of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
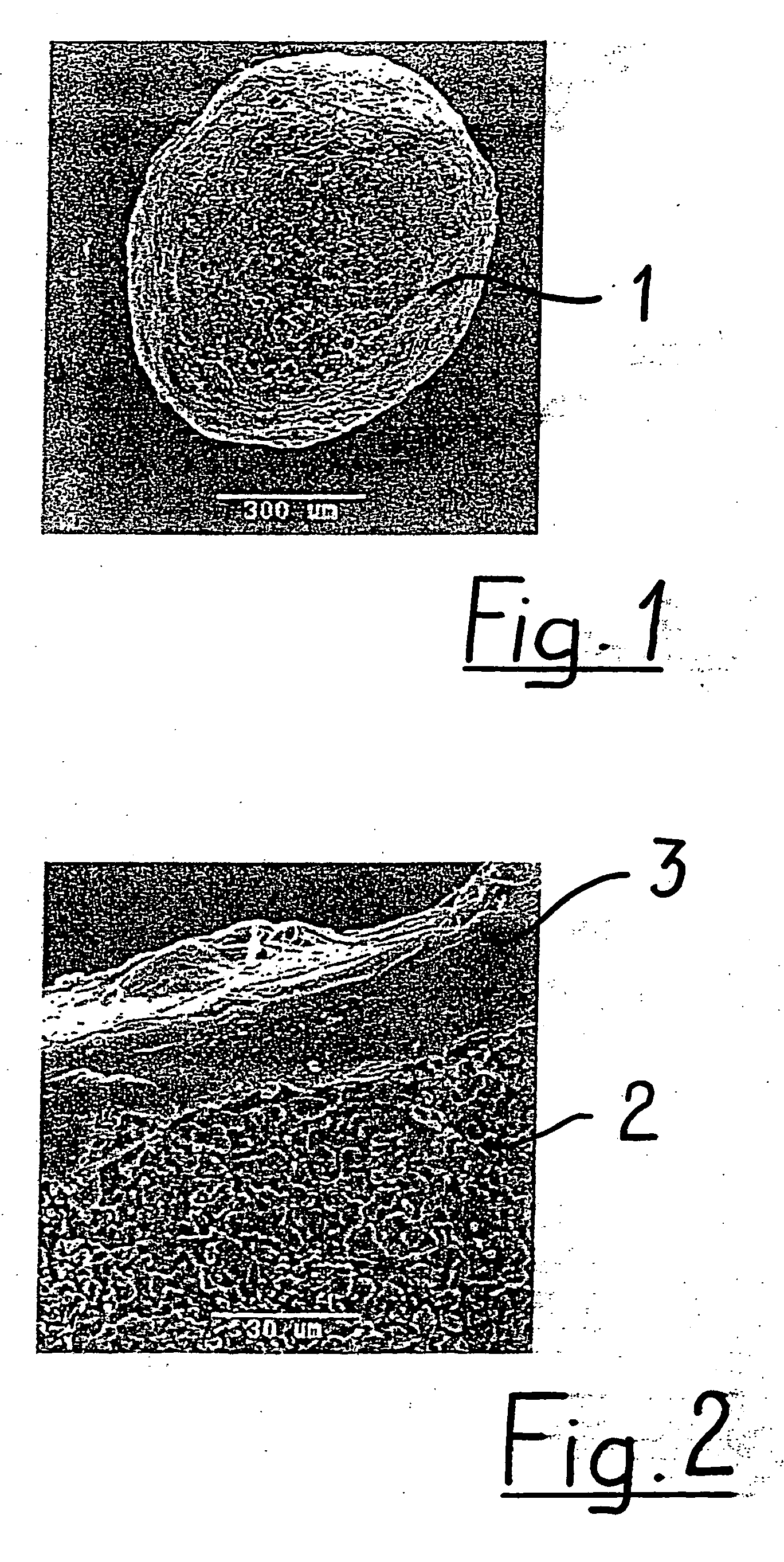
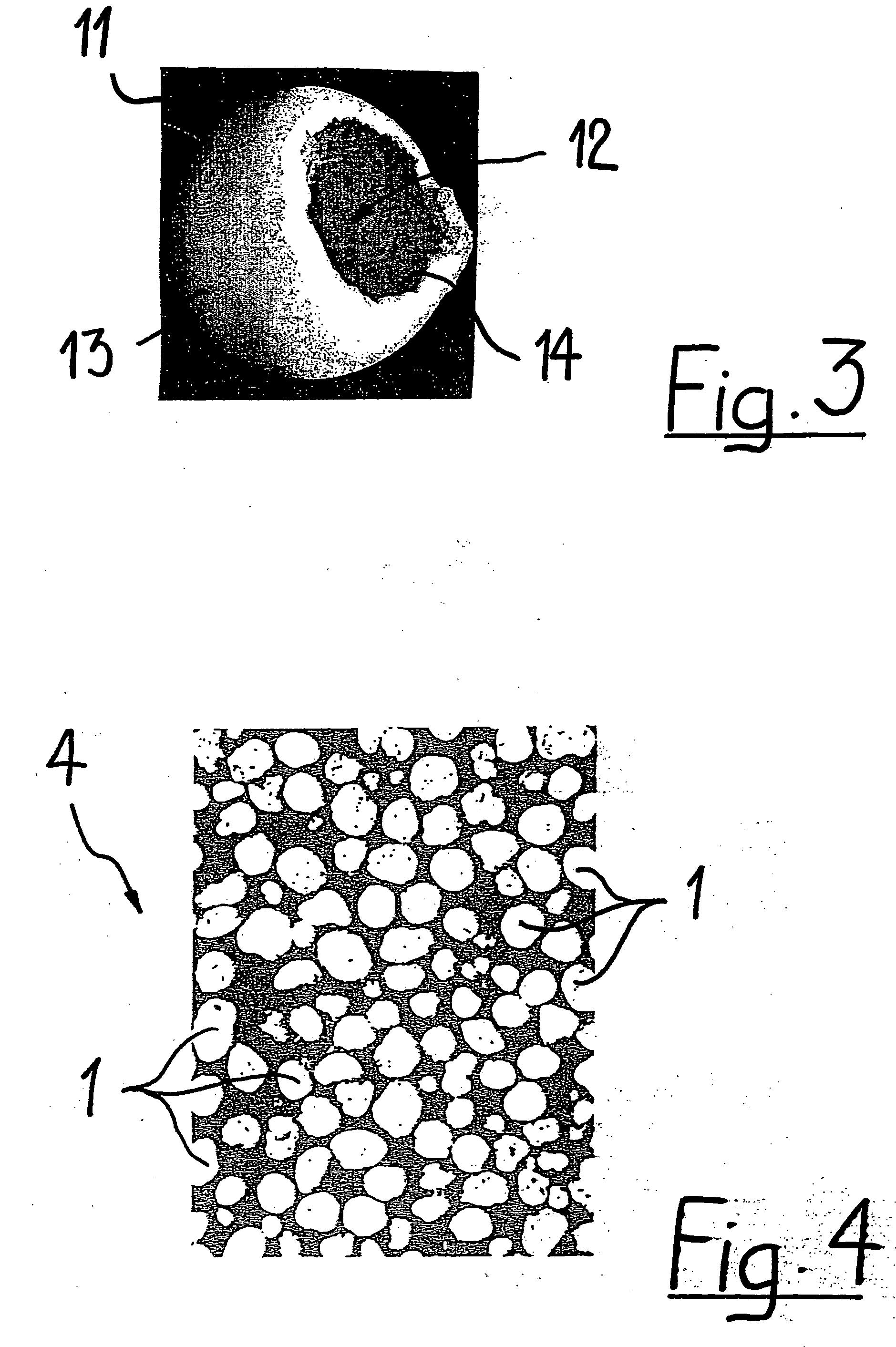
Porous biocompatible implant material and method for its fabrication
a biocompatible and biodegradable implant for a cavity in a bone of a living organism is made of a biocompatible and biodegradable granules which are selected from the group including biopolymers, bioglasses, bioceramics preferably calcium sulfate, calcium phosphate such as monocalcium phosphate monohydrate, monocalcium phosphate anhydrous, dicalcium phosphate dihydrate, dicalcium phosphate anhydrous, tetracalcium phosphate, calcium orthophosphate phosphate, α-tricalcium phosphate, β-tricalcium phosphate, apatite such as hydroxyapatite, or a mixture thereof. The biocompatible and biodegradable granules are provided with a coating, which comprises at least one layer of a biocompatible and biodegradable polymer which is selected from the group including poly((α-hydroxyesters), poly(orthoesters), polyanhydrides, poly(phosphazenes), poly(propylene fumarate), poly(ester amides), poly(ethylene fumarate), polylactide, polyglycolide, polycaprolactone, poly(glycolide-co-trimethylene carbonate), polydioxanone, co-polymers thereof and blends of those polymers. The biocompatible and biodegradable implants are obtained by fusing together the polymer-coated granules through polymer-linkage of the polymer coatings of neighboring granules.
Owner:COLLAGEN MATRIX
Reinforced absorbable synthetic matrix for hemostatic applications
The present invention is directed to a reinforced absorbable hemostat comprising at least one hemostatic agent in a single layer of nonwoven synthetic fabric having a mixture of compressed fiber staples of a polyglycolide / polylactide copolymer and a polydioxanone.
Owner:ETHICON INC
Reinforced Absorbable Synthethic Matrix for Hemostatic Applications
The present invention is directed to a reinforced absorbable hemostat comprising at least one hemostatic agent in a single layer of nonwoven synthetic fabric having a mixture of compressed fiber staples of a polyglycolide / polylactide copolymer and a polydioxanone.
Owner:ETHICON INC
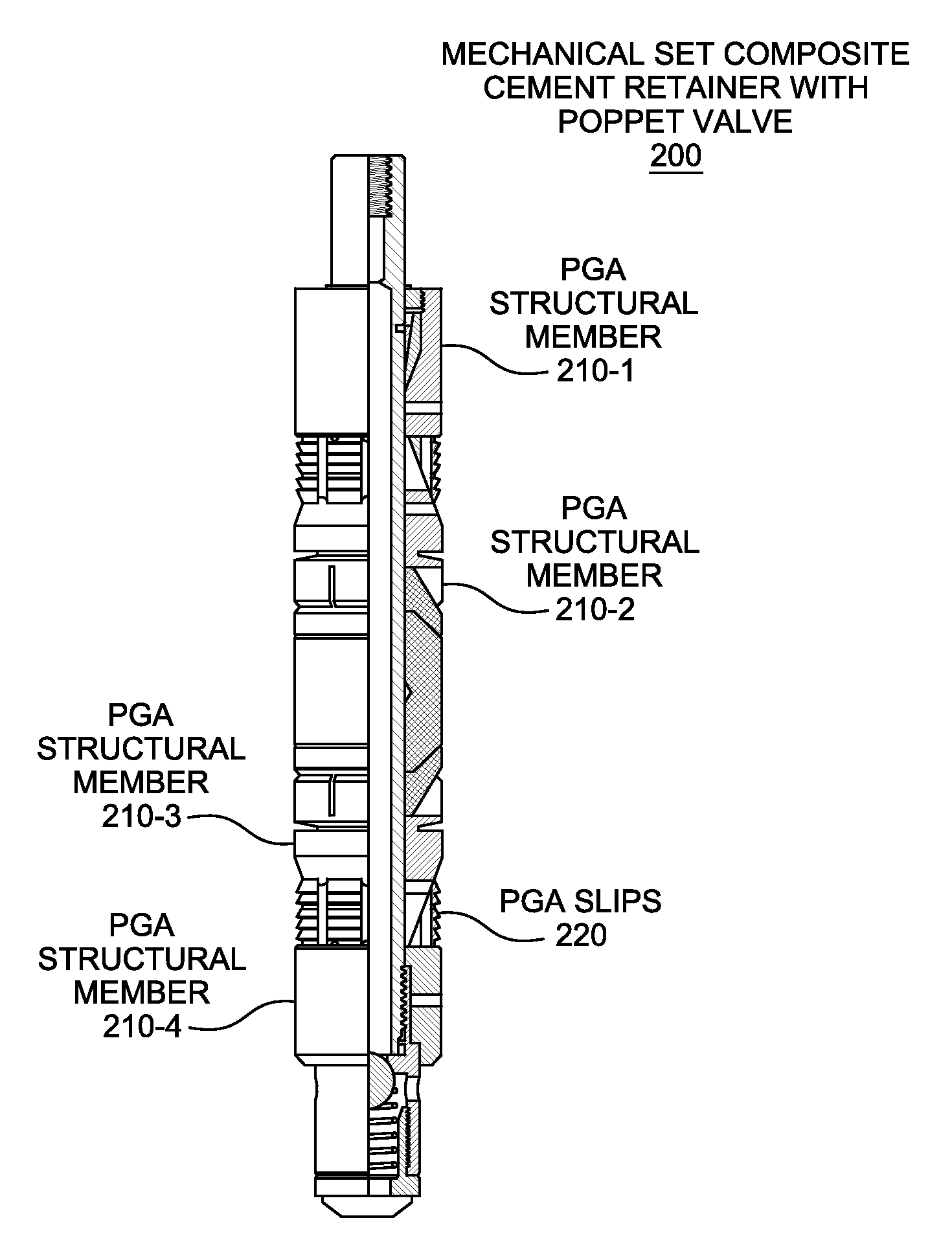
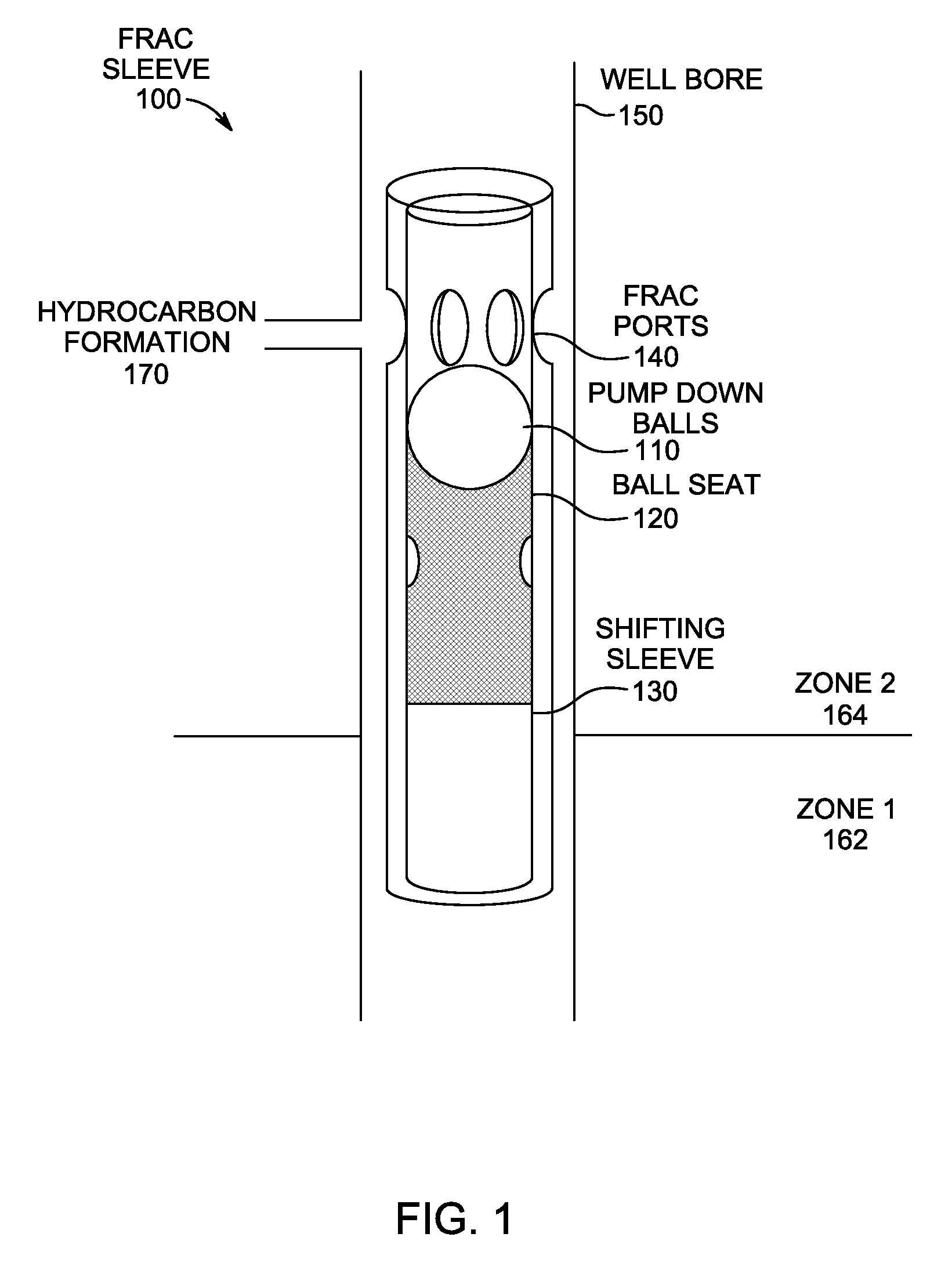
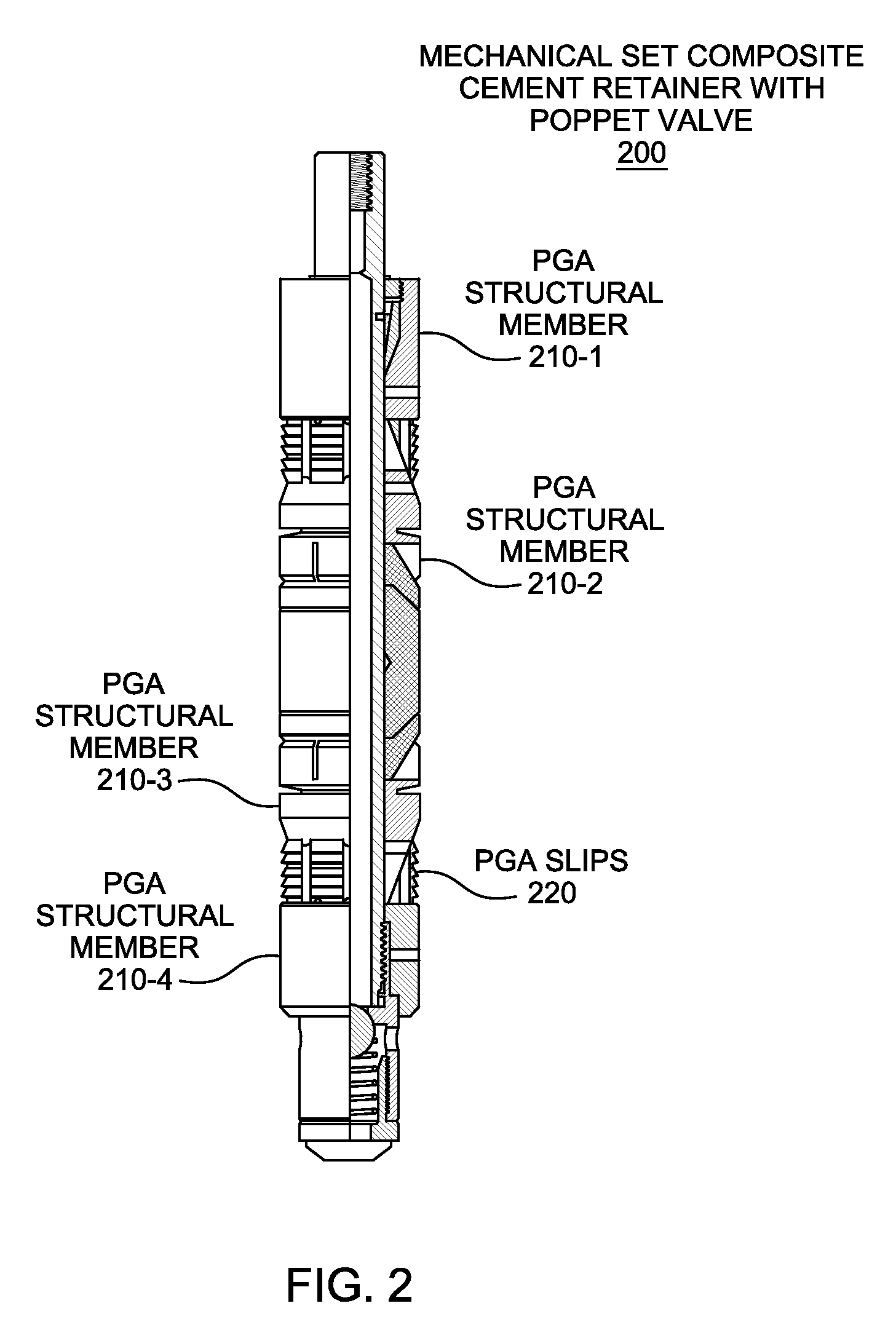
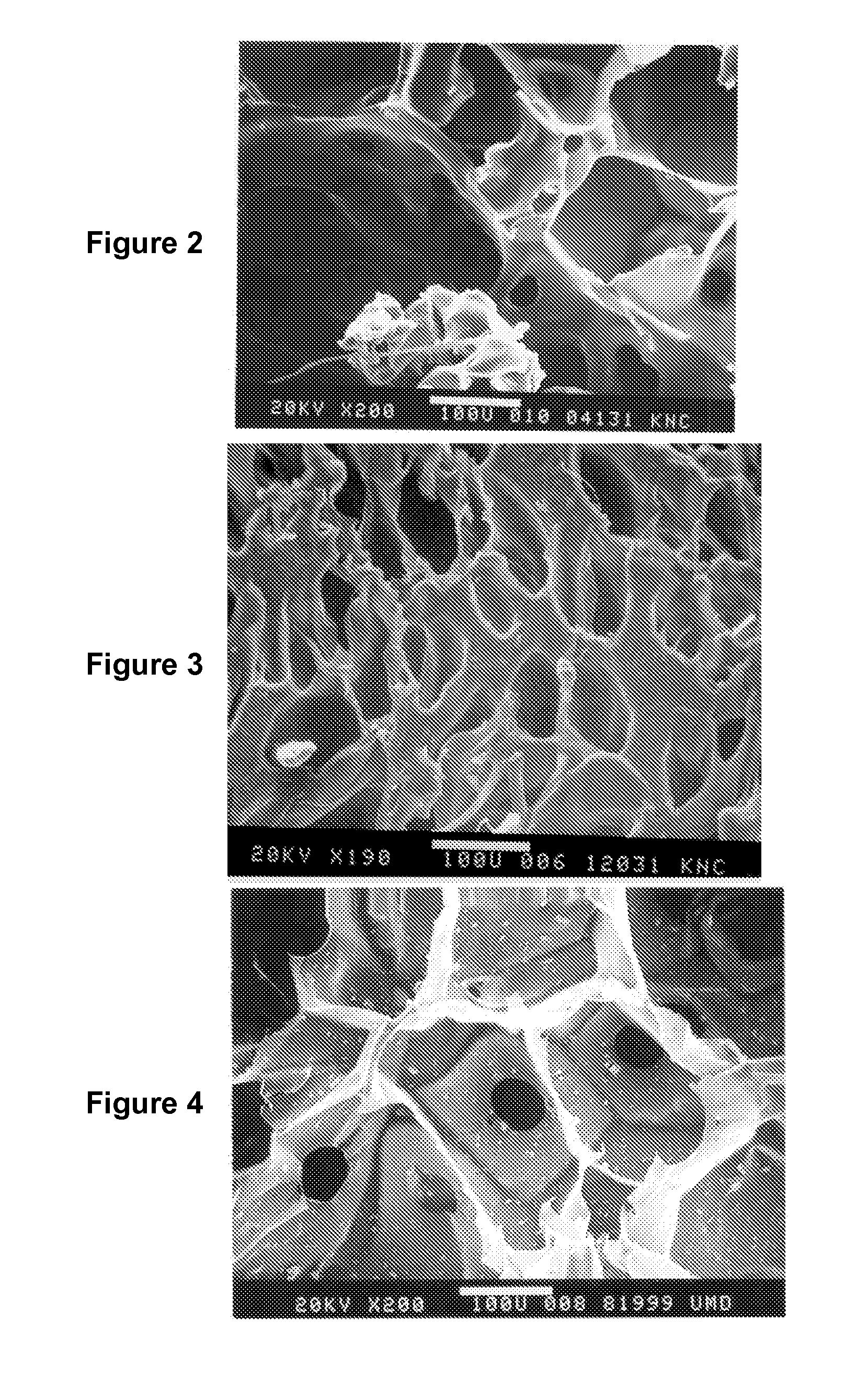
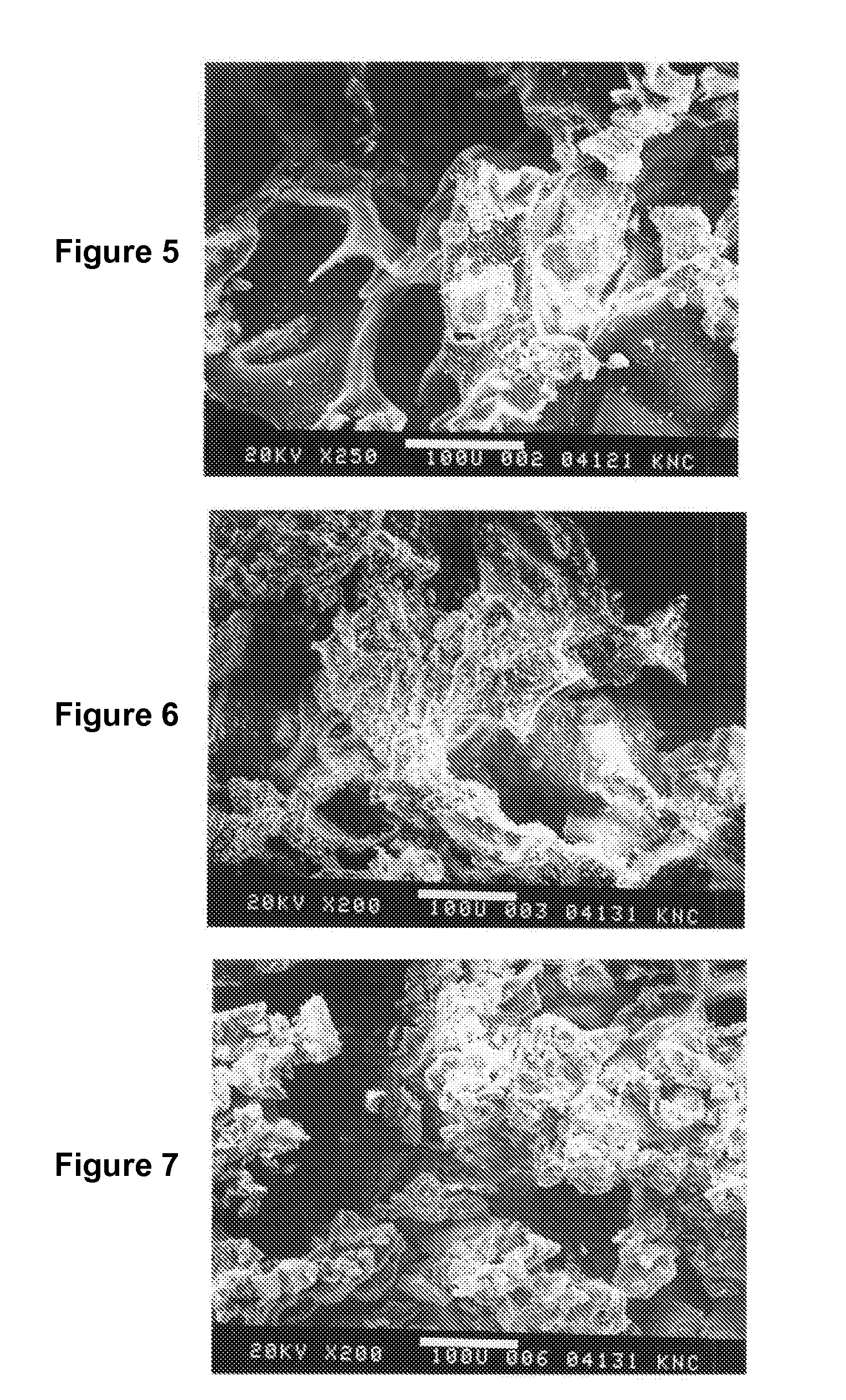
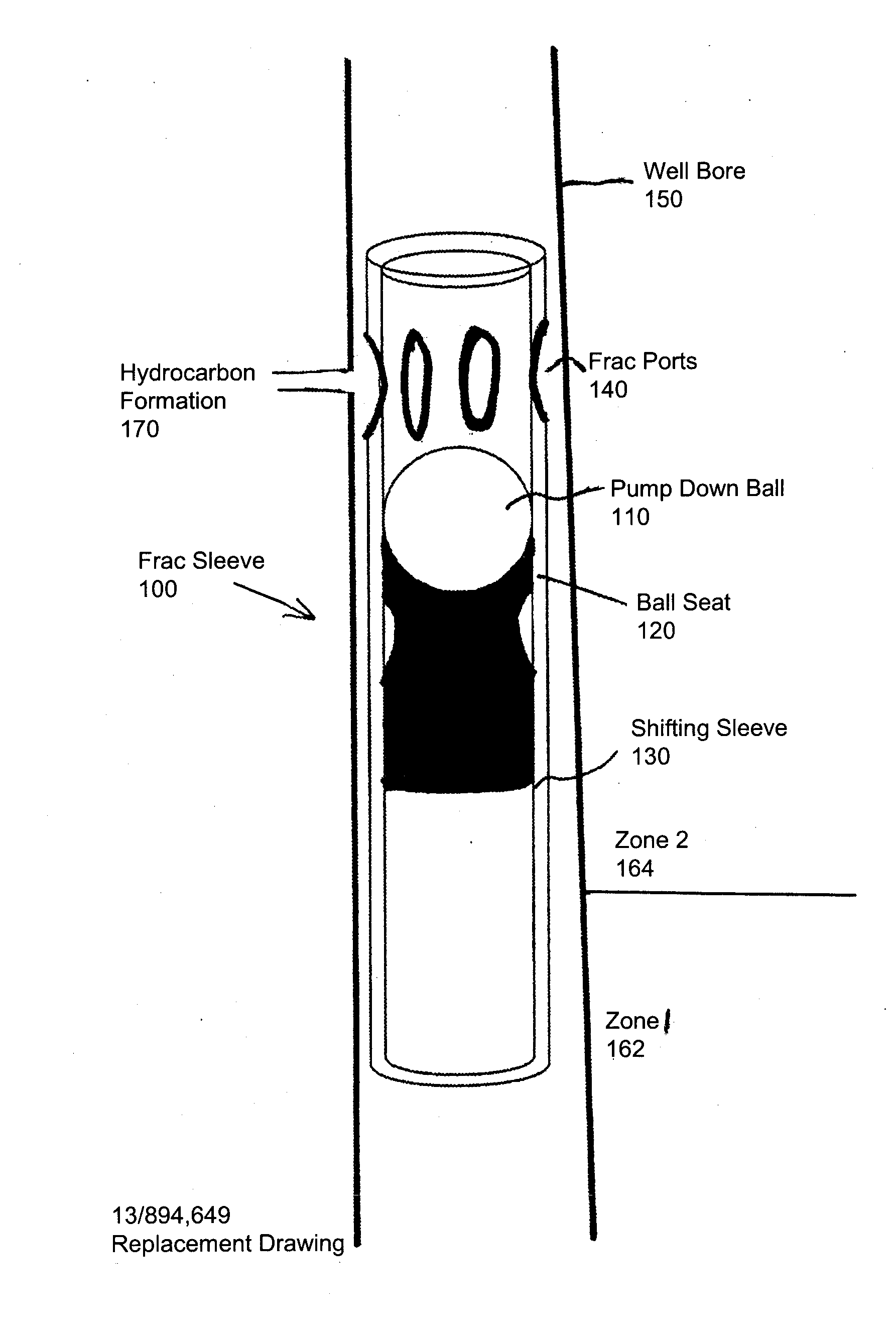
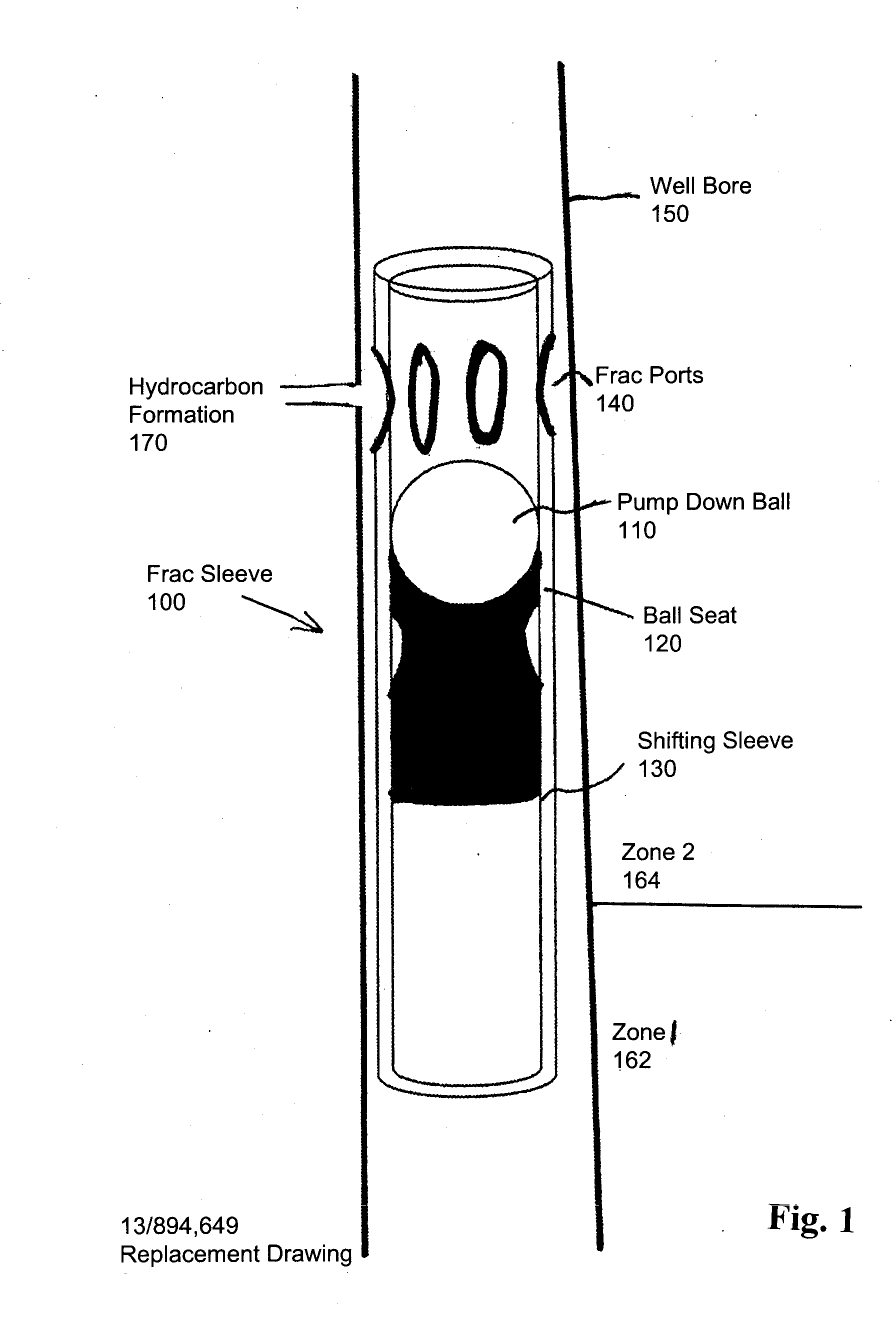
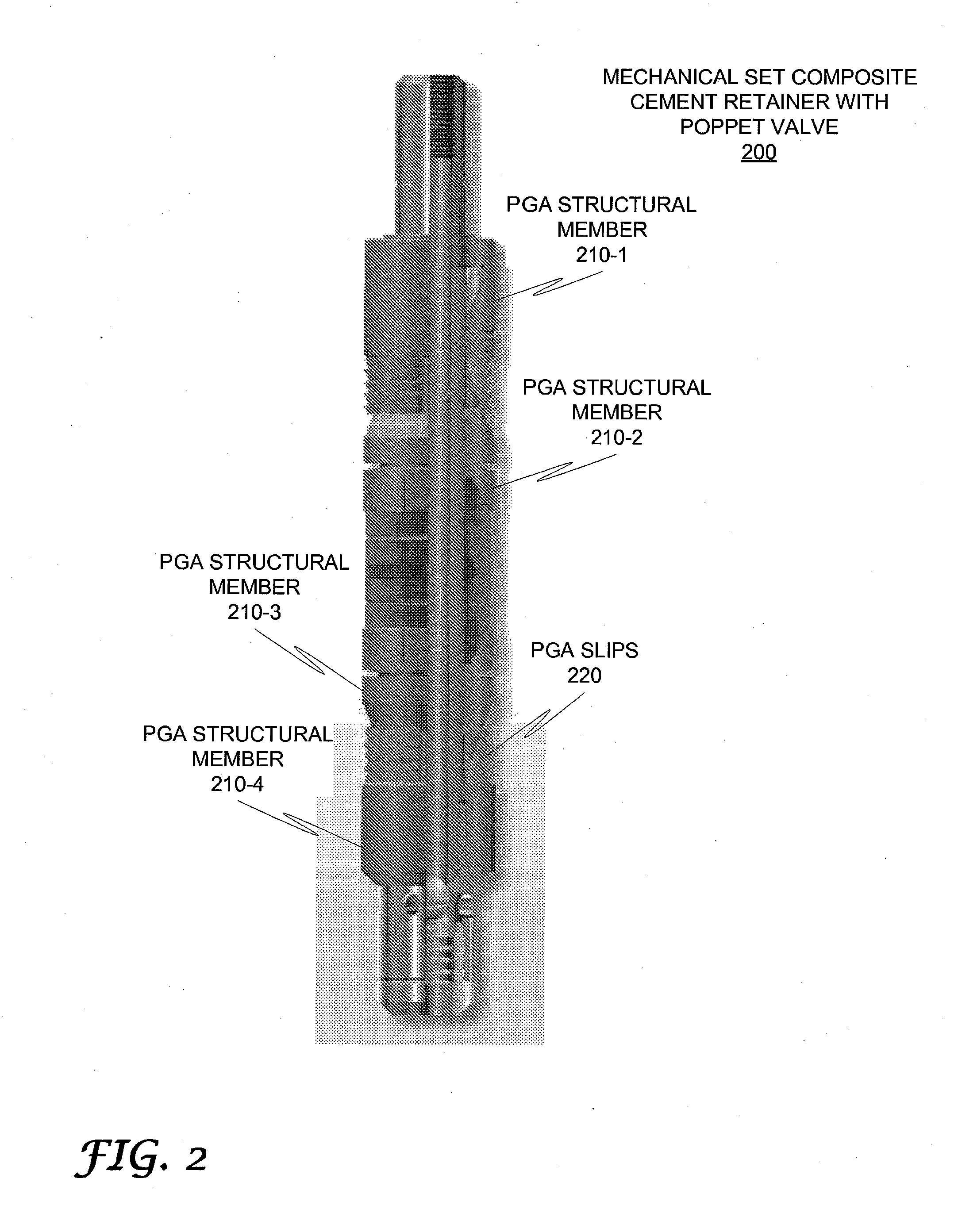
High-molecular-weight polyglycolides for hydrocarbon recovery
A tool having a high-molecular weight Polyglycolides such as polyglycolic acid (PGA) may be used in downhole hydrocarbon recovery applications. Advantageously, PGA tools do not need to be drilled out but will naturally break down into environmentally-compatible natural compounds.
Owner:NINE DOWNHOLE TECHNOLOLGIES LLC
Adsorbable hemostatic ligation clip
ActiveCN101081310AGood compatibilityImprove hydrophilicitySuture equipmentsWound clampsP-dioxanoneHuman body
The present invention discloses one kind of hemostatic ligating clip of absorbable material for surgical abdominoscope operation. The hemostatic ligating clip includes one outer layer of polyglycolide and one inner layer of poly-p-dioxanone or p-dioxanone-lactide copolymer. The hemostatic ligating clip made of materials with high hydrophilicity and flexibility has high compatibility to skin and tissue, no injury to the tissue of human body and the degradation speed determined by the outer layer.
Owner:HANGZHOU SUNSTONE TECH CO LTD +1
High-stability polyethylene glycol-polyester polymer and application thereof
ActiveCN103601878AImprove hydrophobicityHigh drug loadingPharmaceutical non-active ingredientsPolyesterPolymer science
The invention belongs to the field of medical technology, relates to a high-stability polyethylene glycol-polyester polymer and an application thereof, and particularly relates to an amphiphilic block copolymer having a hydrophilic block and a hydrophobic block with terminal hydroxyl, wherein the terminal hydroxyl of the hydrophobic block is replaced by a cholic acid group. The hydrophilic A block is any one of ethylene glycol monomethyl ether, polyethylene glycol, polyvinyl alcohol and polyvinylpyrrolidone; the hydrophobic B block is any one of polylactide, polylactide-co-glycolide, polyglycollide, polycaprolactone, polylactide-co-caprolactone, polyglycollide-co-caprolactone and polylactide-co-glycolide-cocaprolactone. The polymer provided by the invention can spontaneously form high-stability micelle in a waterborne medium, and can be used as a carrier of various water-insoluble drugs.
Owner:SHENYANG PHARMA UNIVERSITY
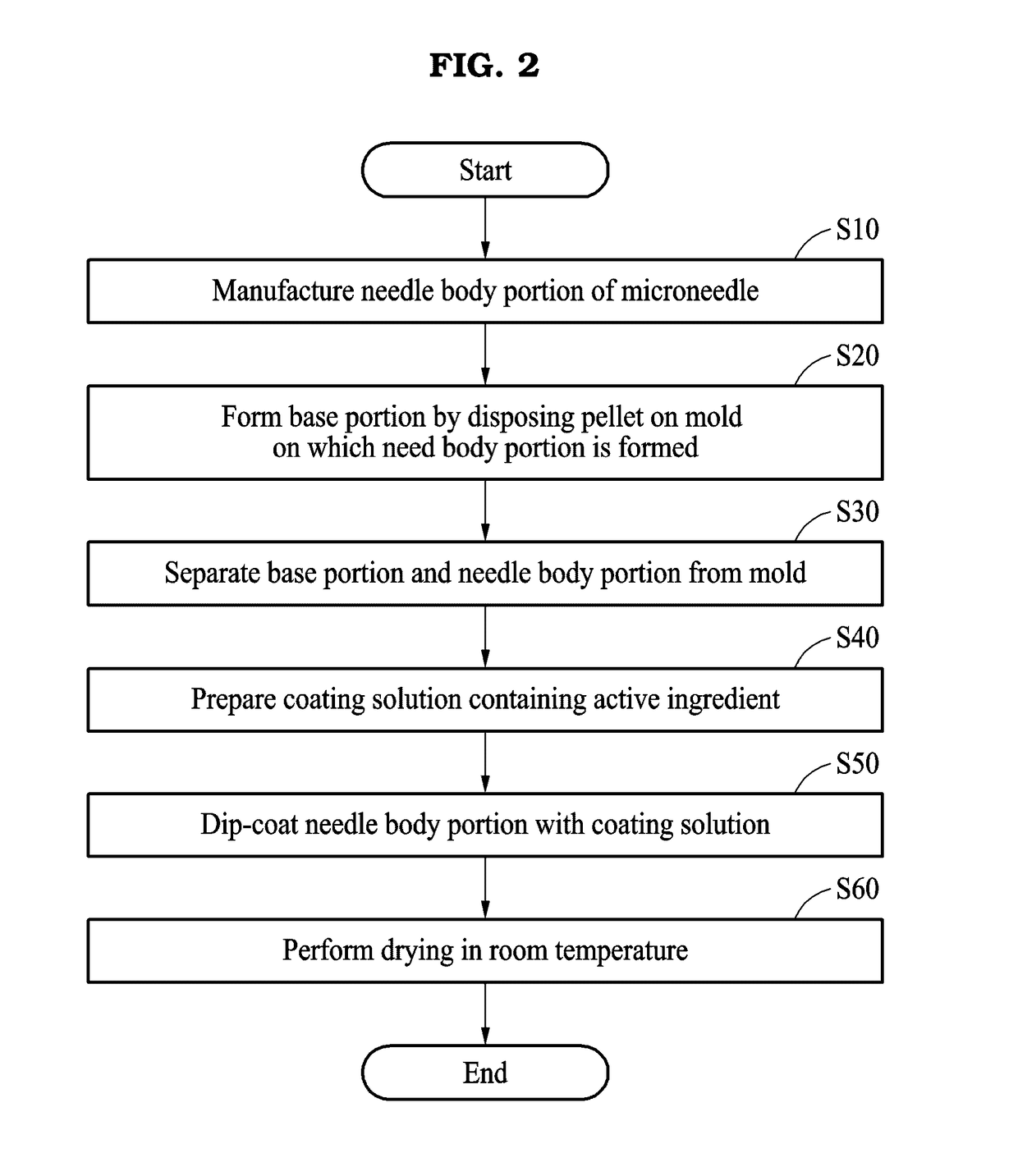
Flexible microneedle for dental material delivery and method of manufacturing the same
ActiveUS20170080196A1Fast deliveryMinimal numberMicroneedlesSurgeryPolyethylene terephthalate glycolPoly(lactide)
Disclosed is a microneedle for dental material delivery, the microneedle including a needle body portion; an active ingredient coating portion configured to coat the surface of the needle body portion, and including an active ingredient transferred to the skin tissue within a mouth; and a base portion configured to couple with the needle body portion, and to bend along a skin shape within the mouth, wherein the base portion includes at least one of polyethylene (PE), polypropylene (PP), polytetrafluoroethylene (PTFE), polymethylmethacrylate (PMMA), ethylene vinyl acetate (EVA), polycaprolactone (PCL), polyurethane (PU), polyethylene terephthalate (PET), polyethylene glycol (PEG), polyvinyl alcohol (PVA), poly lactide (PLA), poly lactic-co-glycolic acid (PLGA), and polyglycolide (PGA).
Owner:BNL BIOTECH +1
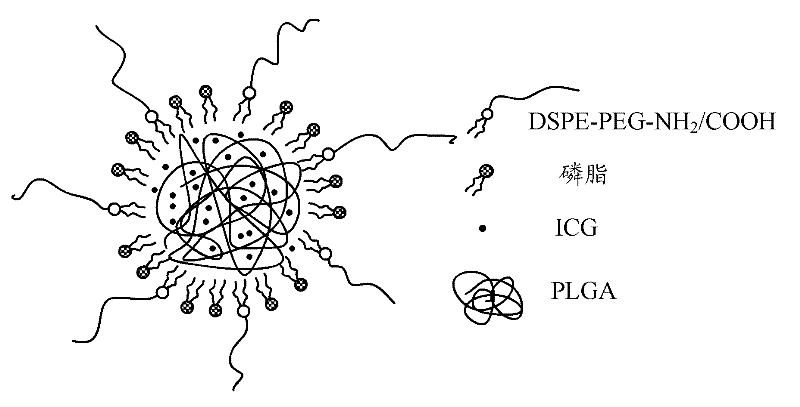
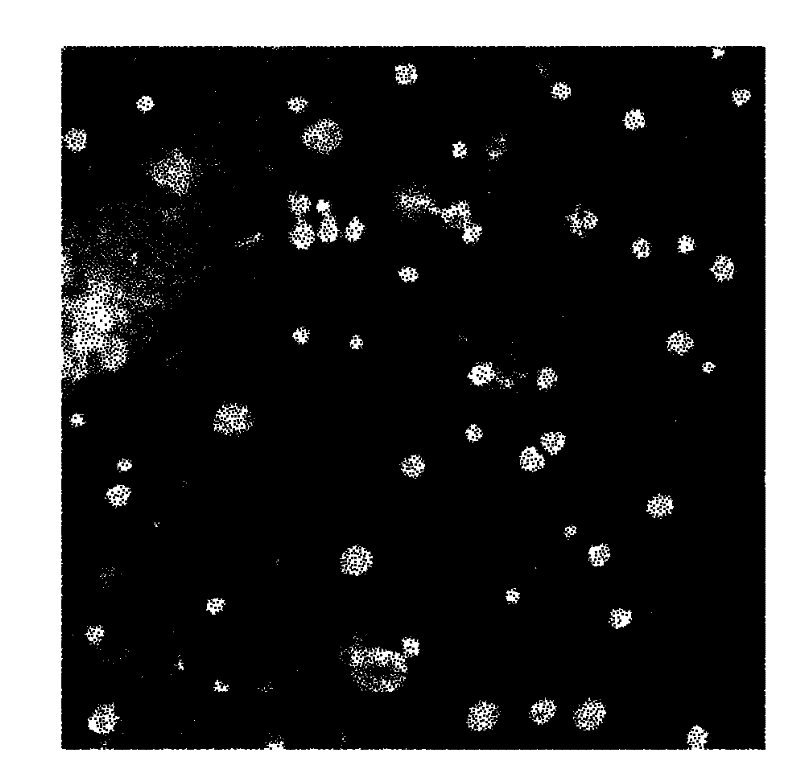
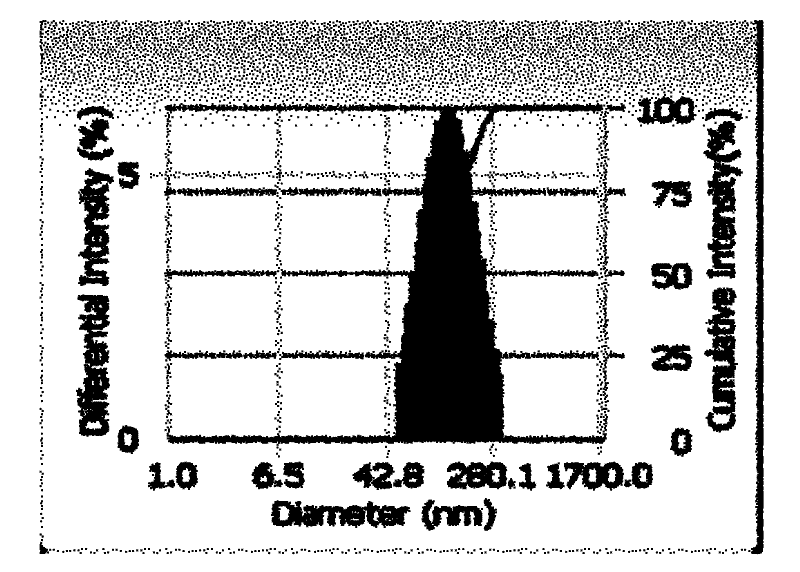
Fluorescence nanometer probe and preparation method thereof
The invention relates to a fluorescence nanometer probe, comprising an inner core formed by polyglycolide lactide, a middle layer formed by phospholipid surrounding on the surface of the inner core and a shell formed by distearoyl phosphatidyl ethanolamine-polyethylene glycol which contains amino or carboxyl and partially penetrates through the middle layer, wherein indocyanine green is dispersed in the inner core. According to the invention, a core-shell structure is formed to ensure that the indocyanine green is wrapped in the polyglycolide lactide, therefore, the indocyanine green is effectively avoided from being aggregated and decomposed and the stability is increased; and the wrapped indocyanine green has a near-infrared fluorescence characteristic to ensure that the background fluorescence penetrating tissues is small and then can be relatively accurately applied to bioluminescence labeling.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Methods and Compositions for Tissue Augmentation
InactiveUS20080075749A1Avoid intakeInhibit migrationCosmetic preparationsPowder deliveryParticulatesPolyolefin
Methods and compositions for use in tissue volume replacement are provided. The present invention comprises compositions comprising a combination of materials, comprising preferably a solid polymer particle phase and a gel phase, and also comprises single phase compositions. More particularly, preferred embodiments comprise a solid polymer particle phase made of materials comprising Gore-Tex (micronized e-PTFE), PDS II (polydioxanone, a monofilament), NUROLON (a long chain aliphatic polymer Nylon 6 or Nylon 6,6) ETHILON (a long chain aliphatic polymer Nylon 6 and Nylon 6,6), PROLENE (Polypropylene, isotactic crystalline stereoisomer of polypropylene, a synthetic linear polyolefin.), VICRYL (copolymer made from 90% glycolide and 10% L-lactide), silk, MONACRYL (poly ε-caprolactone.), polylactide, polyglycolide, poly lactide-co-glycolide, and BIOPOL (polyhydroxyvalerate), MEDPOR (biocompatible (micronized) polyethylene), BIOGLASS (bioactive glass particulate), NOVABONE and NOVABONE-CM, and the gel phase comprises polyvinylpyrrolidone (PVP). Preferred single phase compositions comprise PVP. Methods of the present invention comprising injection of such compositions for tissue augmentation.
Owner:DYER WALLACE K
Use of stannous benzoate as catalyst
ActiveCN1806919AReduce dosageLow cracking temperatureOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBenzoic acidPolymer science
The invention provides the usage of using the benzoic acid stannous as catalyst. The invention solves the problem that polymerization rate of current catalyst in lactic acid and glycolic acid is slow. The benzoic acid stannous is the catalyst used for preparing low-molecular polymer lactic acid with lactic acid dewatering; benzoic acid stannous is the catalyst used for preparing lactide with low-molecular polymer lactic acid cracking; benzoic acid stannous is the catalyst used for preparing polylactic acid with lactide polymerizing; benzoic acid stannous is the catalyst used for preparing low-molecular polymer glycolic acid with glycolic acid dewatering; benzoic acid stannous is the catalyst used for preparing glycolide with low-molecular polymer glycolic acid cracking; benzoic acid stannous is the catalyst used for preparing poly-glycollide with glycolide polymerizing. The benzoic acid stannous is the catalyst used for polymerizing glycolide, lactide and epsilon-caprolactone. Using the benzoic acid stannous is low content, high productivity, high product purity, low temperature and good repeatability.
Owner:ZHEJIANG HISUN BIOMATERIALS +1
Biological degradable shape memory polymer and its preparation process
A biodegradable shape memory polymer is prepared through synthesizing poly-L-lactide oligomer, synthesizing poly(glycolide / caprolactone) cooligomer and using diisocyanate-type coupling agent to couple them together to obtain polyblock copolymer. Its advantage is high effect to hold the thermal deformation and thermally restoring the original shape.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Process and apparatus for recovering lactide from polylactide or glycolide from polyglycolide
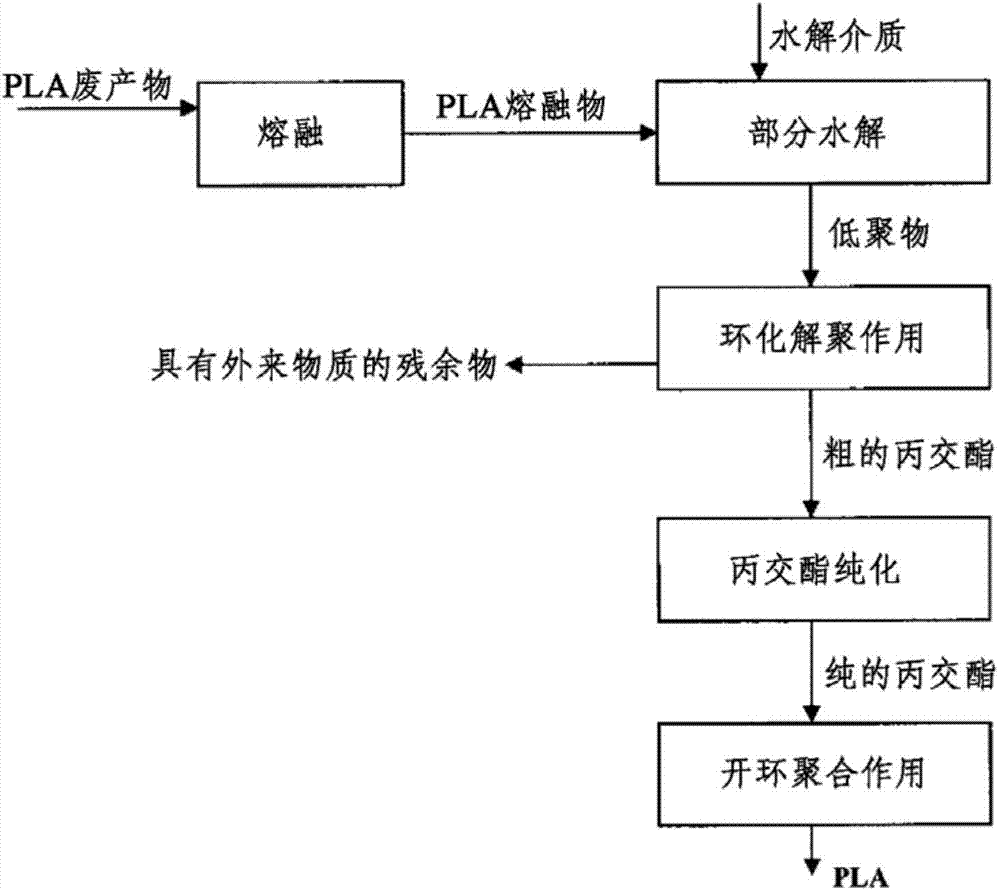
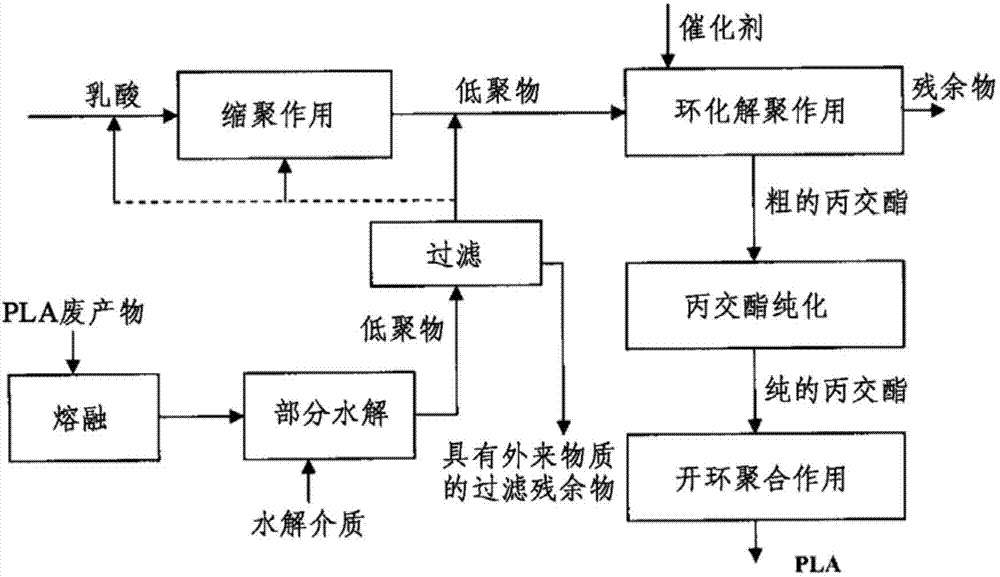
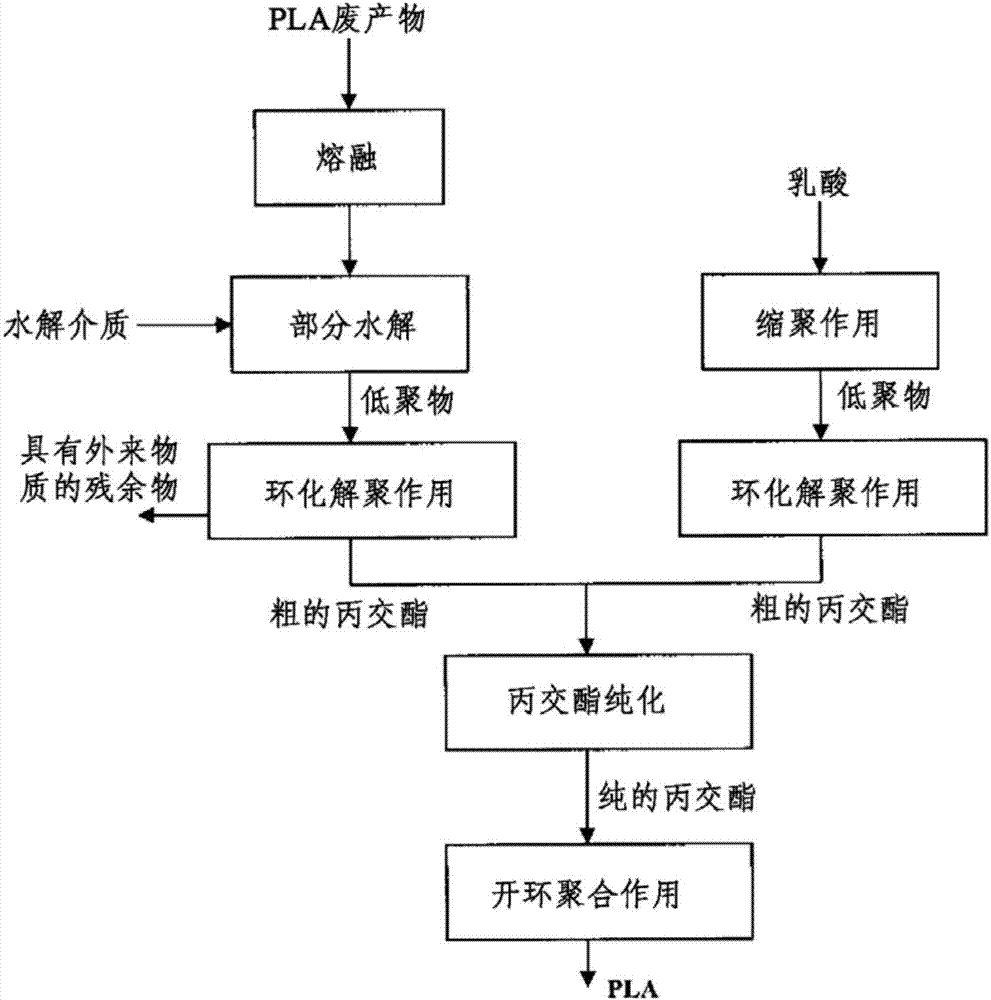
ActiveCN103781833ASave energySaverPlastic recyclingPreparation from carboxylic acid esters/lactonesPartial hydrolysisOligomer
The present invention relates to a process for recovering lactide from polylactide (PLA) or glycolide from polyglycolide (PGA), in which, in a first step, PLA or PGA is contacted with a hydrolysing medium and hydrolytically degraded to oligomers. In a further step, a cyclising depolymerisation of the oligomers obtained in the first step is effected to give lactide or glycolide. In addition, the present invention relates to an apparatus based on the combination of a hydrolysis apparatus with a depolymerisation reactor, with which the above-described process can be performed. The core of the process according to the invention is a partial hydrolysis of the polymeric materials originally used in combination with a cyclising depolymerisation.
Owner:UHDE INVENTA FISCHER
Solution jet method modified antibacterial degradable nano fibers and application thereof in air filtering
The invention discloses a solution jet method modified antibacterial degradable nano fibers and application thereof in air filtering. A preparation method of the nano fibers includes: using one or more of biodegradable polymer such as PLA (polylactic acid), PVA (polyvinyl alcohol), PEO (polyoxyethylene), PEG (polyethylene glycol), PLGA (polylactic acid-glycolic acid copolymer) or PGA (polyglycollide) as the raw materials, sufficiently dissolving the raw materials in an organic solvent which comprises more of chloroform, acetone or ethanol, adding a nano bacterium removing agent which is optional one of TiO2, Ag2O or MnO2, and evenly stirring and dispersing; using a solution jet spinning liquid feed device to prepare nano fibers, and depositing the modified nano fibers to nonwoven fabric toobtain the modified antibacterial degradable nano fibers. The degradable nano fibers is evident in antibacterial effect, good in filtering effect, biodegradable, environmentally friendly and suitablefor large-scale production.
Owner:江苏科来材料科技有限公司
Gel injection combining molecular targeted drug and cytotoxic drug
ActiveCN104622794AImprove tumor killing abilityImprove efficacyAerosol deliveryOintment deliverySide effectLactide
The invention relates to a gel injection combining a molecular targeted drug and a cytotoxic drug. The gel injection comprises a small molecular targeted drug, a traditional cytotoxic drug, a temperature-sensitive material, a solvent and pharmaceutical excipients, wherein the temperature-sensitive material is selected from poloxamer, polylacticacid-polyethylene glycol-polylactic acid or polyglycolide lactide-polyethylene glycol-polyglycolide lactide and the like; the solvent is selected from water, brine salt or 5% glucose aqueous solution and the like, the prepared gel injection can be injected intratumorally or peritumorally, the anti-tumor effect is obvious, and the side effects are fewer.
Owner:PEKING UNIV
Medicament carrying nano particles and preparation method and application thereof
InactiveCN102370622AGood water dispersibilityImprove solubilityPowder deliveryOrganic active ingredientsGlycolic acidPolyglycolide
The invention discloses medicament carrying polymer nano particles and a preparation method thereof in the technical field of pharmacy. The medicament carrying polymer nano particles contain medicaments and polylactic acid-methoxy polyethylene glycol (PLA-mPEG) or poly (lactic-co-glycolic acid)-methoxy polyethylene glycol (PLGA-mPEG). The molecular weight of the block polylactic acid (PLA) or the poly (lactic-co-glycolic acid) (PLGA) is 10,000 to 60,000, the molecular weight of the block methoxy polyethylene glycol (mPEG) is 2,000 to 5,000, and the block ratio of the PLA or the PLGA to the mPEG is (60:40)-(90:10). The preparation method of the nano particles avoids the defects of low medicament nano particle entrapment rate and serious burst release caused by using the conventional surfactant, complex removing steps and the like; and the medicament carrying polymer nano particles with high quality are synthesized by simplified processes and with relatively low cost.
Owner:ZHEJIANG UNIV
Triblock copolymer using furandicarboxylic acid flexible random copolyester as soft block and preparation method thereof
The invention discloses a triblock copolymer using furandicarboxylic acid flexible random copolyester as a soft block and a preparation method thereof. The triblock copolymer is of a hard-soft-hard structure, the mass ratio of the soft block to the hard block is (10:90)-(90:10); the hard block is polylactic acid, polyglycolide or poly (p-dioxanone); and the soft block is a random copolyester composed of a furandicarboxylic acid dibasic alcohol ester chain element and at least one aliphatic dibasic acid dibasic alcohol ester chain element. The triblock copolymer is obtained by firstly carrying out copolycondensation on furandicarboxylic acid, at least one aliphatic dibasic acid and aliphatic dibasic alcohol to obtain a hydroxyl-terminated furandicarboxylic acid copolyester soft block and then initiating ring-opening polymerization of lactide, glycolide or p-dioxanone. The triblock copolymer is a partial or complete bio-based polymer, the hard block is crystallizable and the soft block has weak crystallinity or is close to be amorphous and has excellent comprehensive performances. The preparation method disclosed by the invention has the advantages of simple process, no application of organic solvents and environmental friendliness and is conductive to commercialization.
Owner:ZHEJIANG UNIV
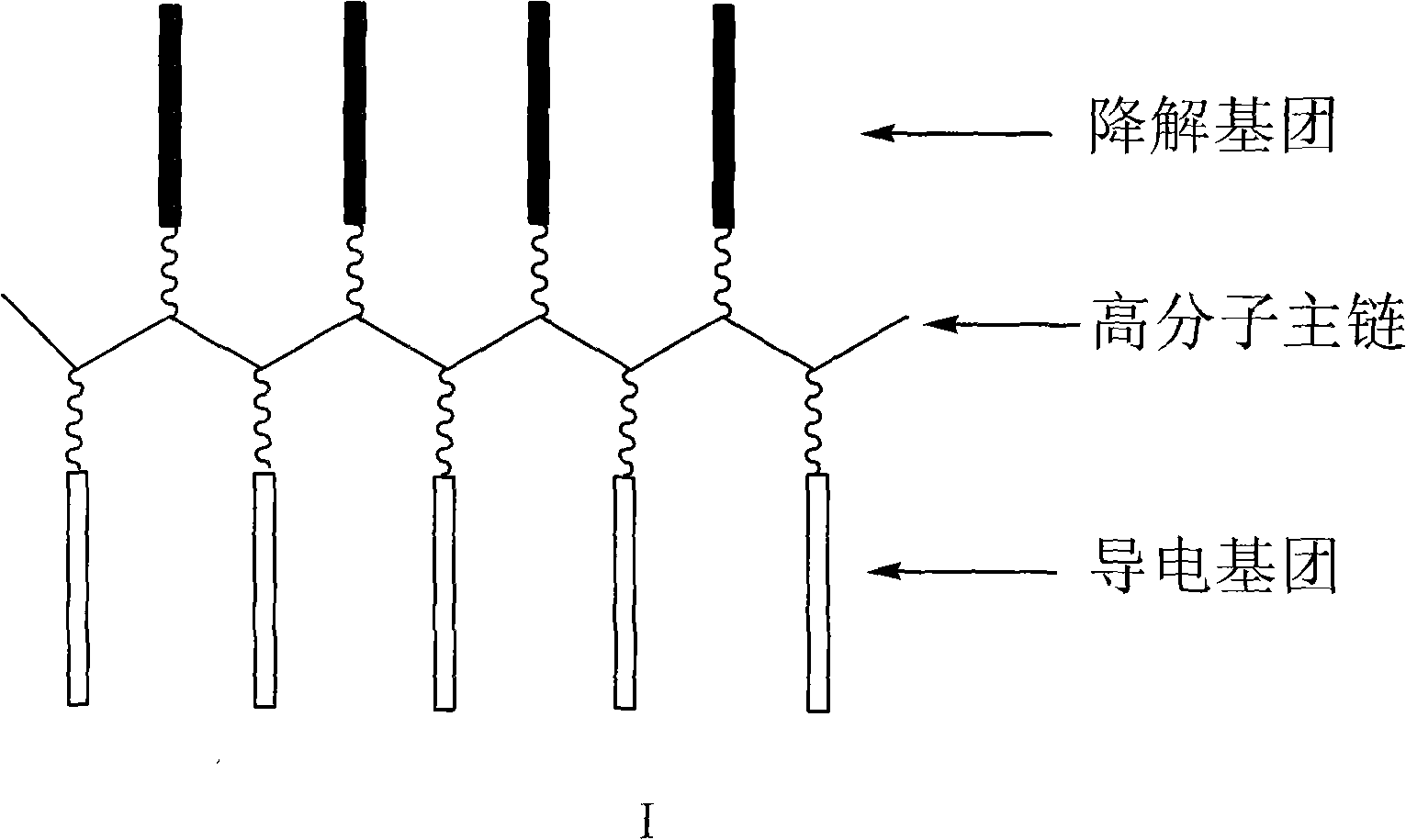
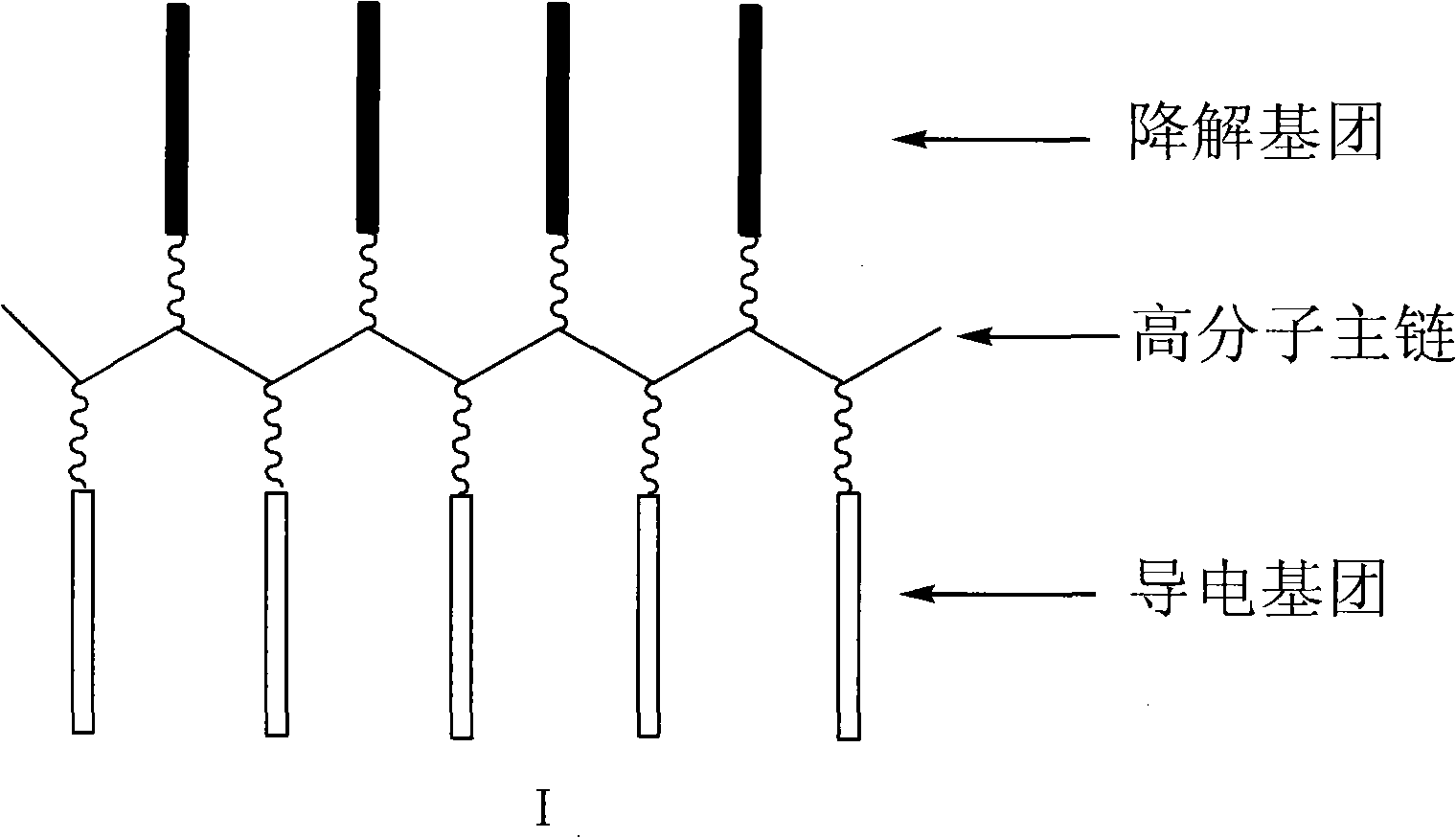
Degradable conductive biological medical polymer material
The invention discloses a degradable electricity-conducting biomedical polymer material, which has a molecular constitution as shown in formula I: the polymer material takes the polymer in the formula, which comprises polyphosphazene, chitosan, polyvinyl alcohol, poly-beta-malic acid or modified polyester, as the main chain, a conductive group and a degradable group are grafted, and the modified polyester is lactic acid, polyglycolide, polycarbonate, polyanhydride, polycaprolactone or copolymers between each two thereof; the conductive group comprises oligo-pyrrole, oligo-thiophene, oligo-aniline or copolymers between each two thereof; the degradable group mainly comprises: (1) amino: glycine ethyl ester, phenylalanine ethyl ester, imidazole and the like; and (2) alkoxy: glycerol, glucose, lactic acid, p-methyl phenol, glycolic acid and polyester. The polymer material has degradability and conductivity and can be prepared into guide pipes, suture threads, membranes, sheet bodies, block bodies and materials for frames of tissue engineering.
Owner:WUHAN UNIV OF TECH
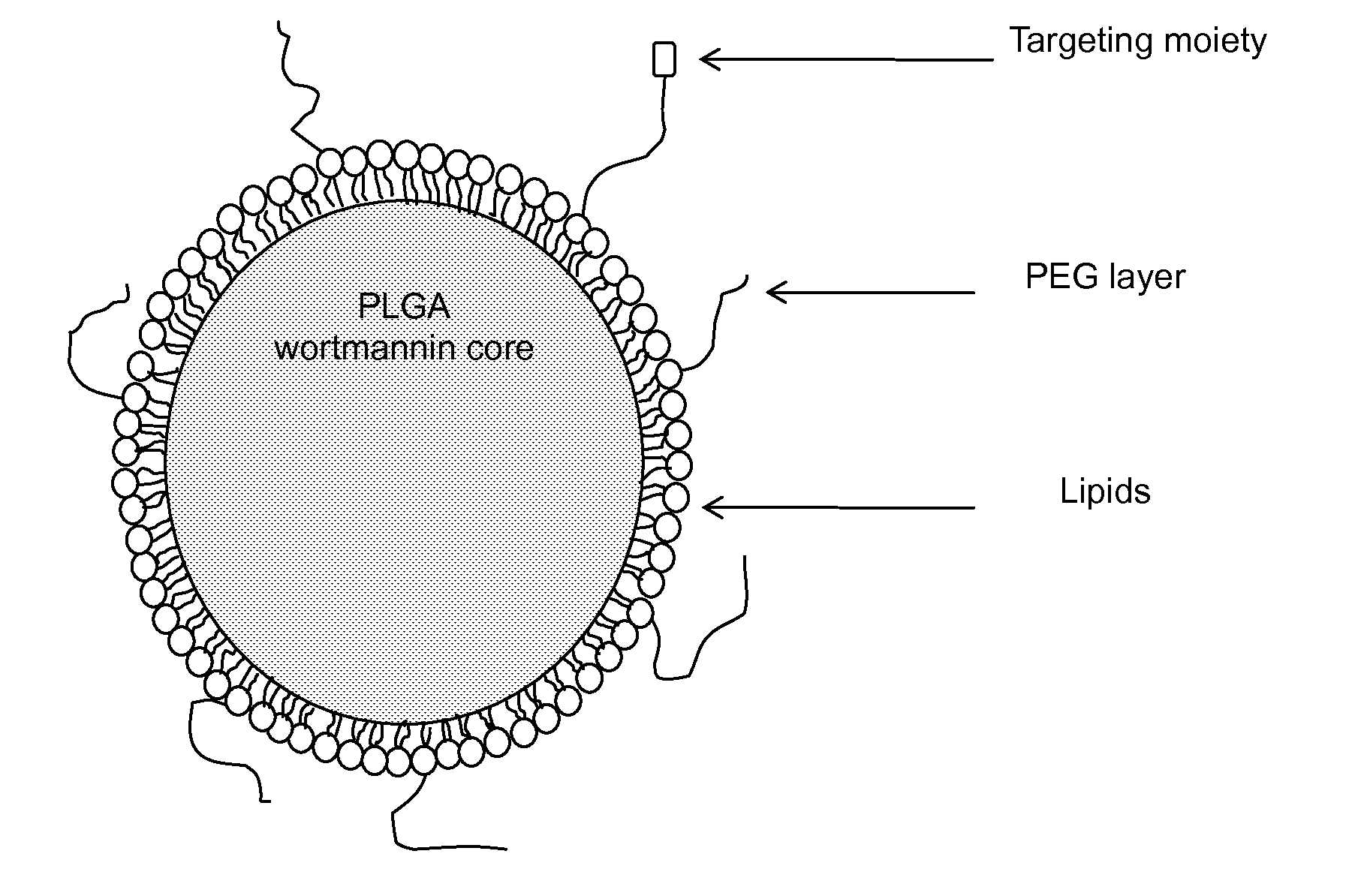
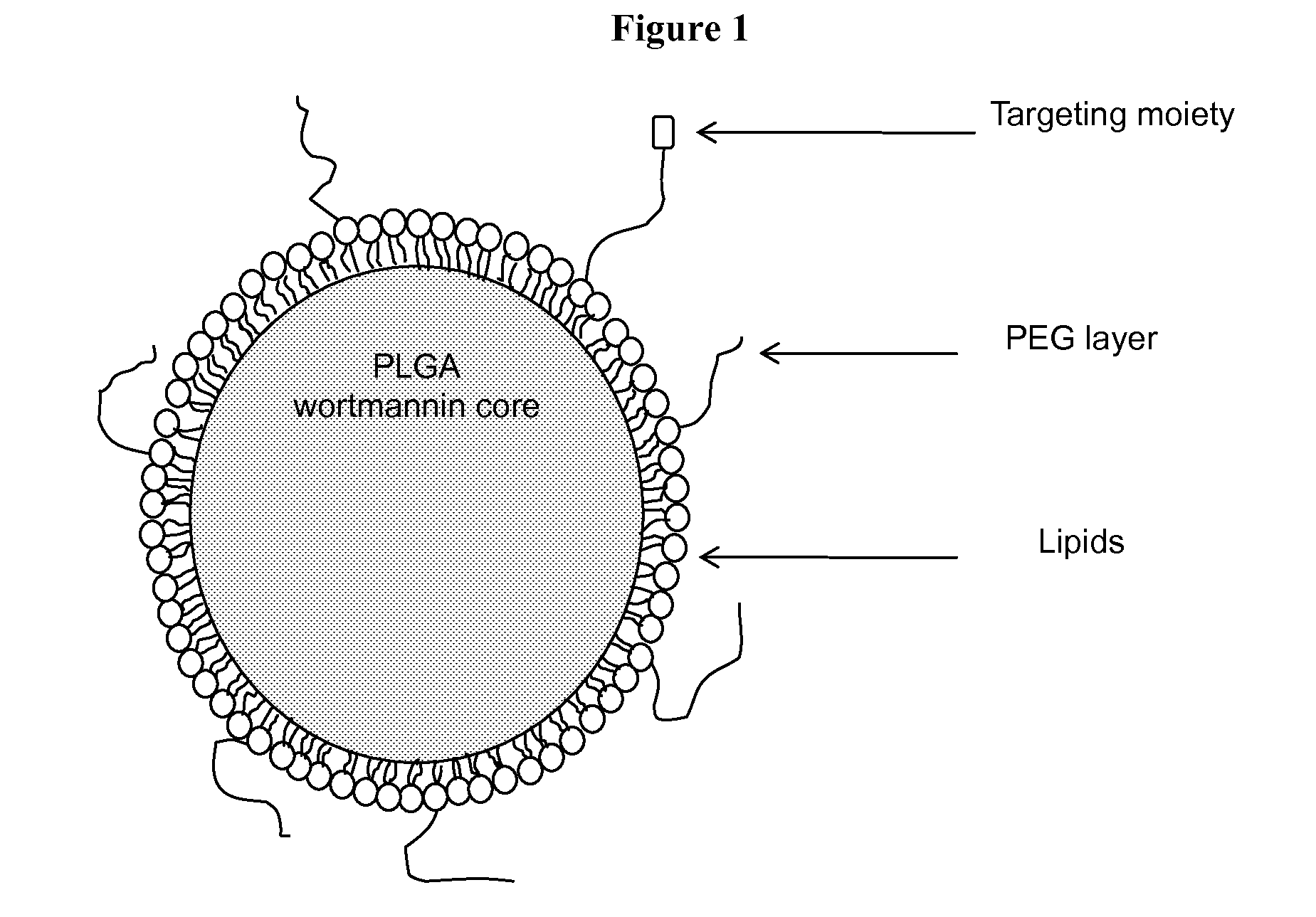
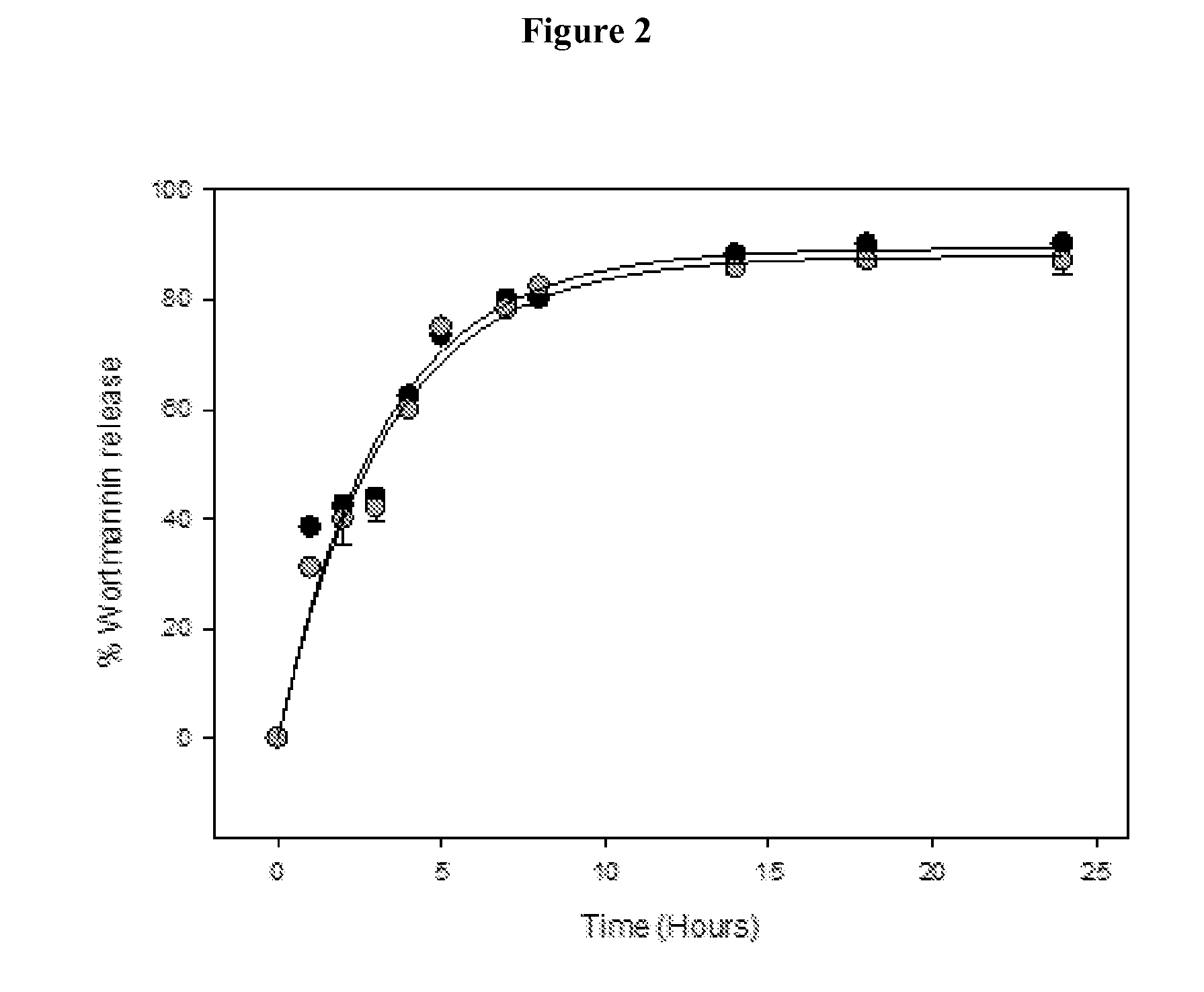
DNA repair enzyme inhibitor nanoparticles and uses thereof
InactiveUS20140017165A1Ultrasonic/sonic/infrasonic diagnosticsBiocidePolyethylene glycolRepair enzyme
This invention relates generally to the discovery of novel nanoparticles for delivery of DNA double-stranded break (DSB) repair enzyme inhibitors such as wortmannin or wortmannin analogues. In one embodiment, these nanoparticles comprise a polylactide polyglycolide (PLGA) copolymer and a polyethylene glycol (PEG). In addition methods of treatment and methods of enhancing radiation treatments are also provided.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Production of moldable bone substitute
InactiveUS20100226956A1Long shapeability timeHigh final strengthInorganic phosphorous active ingredientsSkeletal disorderPorosityLactide
Composites and methods of producing a mouldable bone substitute are described. A scaffold for bone growth comprises nanocrystalline hydroxyapatite (HA), a bioresorbable plasticizer, and a biodegradable polymer. Plasticizers of the invention include oleic acid, tocopherol, eugenol, 1,2,3-triacetoxypropane, monoolein, and octyl-beta-D-glucopyranoside. Polymers of the invention include poly(caprolactone), poly(D,L-Lactic acid), and poly(glycolide-co lactide). Methods of regulating porosity, hardening speed, and shapeability are also described. Composites and methods are described using nanocrystalline HA produced with and without amino acids. The scaffold for bone growth described herein displays increased strength and shapeability.
Owner:PROMIMIC
Resorbable structure for treating and healing of tissue defects
InactiveUS20060210598A1Extended stayPreserve molecular weightSurgeryPharmaceutical delivery mechanismCisplatinTissue defect
Devices and processes (e.g., improved Plasticized Melt Flow processes (PMF) or improved Phase Separation Polymer Concentration (PSPC), etc.) used to make resorbable and non-resorbable structures for treating and / or healing of tissue defects are disclosed. Among the advantages of using these improved processes are the preservation of molecular weight and the broadening of the processing conditions for temperature sensitive polymers and therapies (e.g. polylactide, polyglycolide, polycaprolactone or Cisplatin, etc.). This reduction in processing temperature, pressure and time can help to preserve the molecular weight and / or integrity of the final product or any additive incorporated therein. Additionally, pore size and shape tailoring can increase the osteoconductive nature of the device.
Owner:EVANS DOUGLASG +2
Hydrogel and preparation method thereof
InactiveCN106046398AEasy injectionAdjustable viscosityPeptide/protein ingredientsAerosol deliveryBenzoic acidCross-link
The invention provides hydrogel which comprises the following raw materials in parts by weight: 1-50 parts of chitosan, 0.1-20 parts of a cross-linking agent and 10-100 parts of water, wherein the cross-linking agent comprises the following raw materials in parts by weight: 1-5 parts of a raw material A and 0.1-5 parts of a raw material B; after the raw material A is combined with the raw material B, a raw material C is connected with the final end of the mixture; the raw material A is polyethylene glycol; the raw material B is a degradable polymer; the raw material B is selected from one or more of polyglycollide, polylactide, polyglycollide lactide and polycaprolactone; and the raw material C is selected from one or more of glyoxylic acid, uronic acid and aldehyde benzoic acid. The invention further provides a preparation method of the hydrogel. The hydrogel has the beneficial effects that a reaction product of polyethylene glycol and the degradable polymer is enabled to react with glyoxylic acid, uronic acid and aldehyde benzoic acid, and the obtained cross-linking agent and chitosan form the hydrogel automatically in a solution.
Owner:深圳诺坦药物技术有限公司
High-molecular-weight polyglycolides for hydrocarbon recovery
ActiveUS20140083717A1Crystal structure be loseFluid removalVibration devicesHigh molecular massPolyglycolide
A tool having a high-molecular weight Polyglycolides such as polyglycolic acid (PGA) may be used in downhole hydrocarbon recovery applications. Advantageously, PGA tools do not need to be drilled out but will naturally break down into environmentally-compatible natural compounds.
Owner:NINE DOWNHOLE TECHNOLOLGIES LLC
High-purity glycolide preparation method
The invention provides a high-purity glycolide preparation method, which comprises: preparing a polyglycolide (PGA) oligomer through pressure reducing dehydration, carrying out high-temperature cracking distillation to obtain crude glycolide, and finally purifying to obtain the polymer-grade high-purity glycolide. According to the present invention, the method has characteristics of low reaction conditions, rapid reaction, short reaction time and high purity of the purified glycolide, and can meet the requirements in polymerization of high-molecular-weight polyglycolide.
Owner:SHANDONG GUYUCHUN BIOTECHNOLOGY CO LTD
Reinforced absorbable multi-layered fabric for hemostatic applications
The present invention is directed to a synthetic fabric comprising a multi-layered nonwoven fabric made from staples of a polyglycolide / polylactide copolymer, each layer having a different density. The multi-layer fabric can be used as a reinforced absorbable hemostat medical device.
Owner:ETHICON INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com