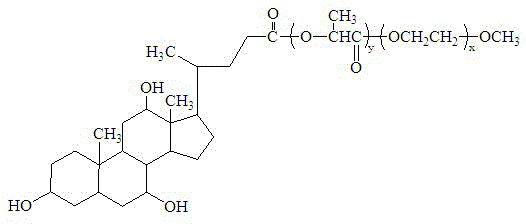
High-stability polyethylene glycol-polyester polymer and application thereof
A polyethylene glycol and high-stability technology, which is applied in the direction of medical preparations and pharmaceutical formulations of non-active ingredients, can solve the problems of toxicity, reduced biodegradability, and insufficient excretion of polymers, and achieve drug loading High, improve targeting, and simplify the effect of industrial production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Synthesis of amphiphilic block copolymer mPEG-PDLLA-CA
[0036] The mPEG-PDLLA diblock copolymer was synthesized by ring-opening polymerization. After the polymer tube is soaked in chromic acid washing solution, it is washed with distilled water and dried for later use. Weigh a certain proportion of polyethylene glycol monomethyl ether (mPEG2000) and lactide monomer into a dry polymerization tube, and then add the catalyst Sn(Oct) 2 (The dosage is 1‰ of the weight of the reactant). After deoxygenation with high-purity nitrogen, the tube was sealed under vacuum and reacted in an oven at 140°C for 6 hours to obtain a light yellow transparent semi-solid product. The obtained crude product was cooled, dissolved with a small amount of dichloromethane and then transferred to a small beaker, precipitated with a large amount of ether in the cold trap, and the crystals were suction filtered. This operation was repeated twice to obtain a relatively pure two-stage pol...
Embodiment 2
[0040] The mensuration of embodiment 2 block copolymer CMC
[0041] (1) Preparation of stock solution
[0042] Preparation of pyrene stock solution: Weigh 6mg of pyrene into a 50mL brown volumetric flask, add acetone to dissolve and dilute to the scale to make 6×10 -4 mol L -1 pyrene solution. Accurately measure 1.0mL into a 25mL volumetric flask, add acetone to constant volume, and obtain a concentration of 2.4×10 -5 mol L -1 Pyrene solution, accurately draw 10mL into a 100mL brown volumetric flask, and dilute with acetone to a final concentration of 2.4×10 -6 mol L -1 , to obtain the stock solution of pyrene.
[0043] Preparation of polymer stock solution: Weigh 20 mg of mPEG-PDLLA-CA block copolymer into a 20 mL volumetric flask, dissolve in distilled water and dilute to the mark to obtain a concentration of 1 mg·mL -1 polymer stock solution.
[0044] (2) Determination of CMC
[0045] Sample preparation: Add 500uL pyrene stock solution to a 10 mL volumetric fla...
Embodiment 3
[0053] Example 3 Preparation and particle size determination of paclitaxel-loaded polymer micelles
[0054] Freeze-drying method: First weigh 7 mg of paclitaxel and dissolve it in tert-butanol, and then mix it with an aqueous solution containing 20 mg of mPEG-PDLLA-CA block copolymer, so that the volume ratio of water / tert-butanol is 70:30. The mixed solution was stirred at 4°C for 3 hours, then sterilized by filtration with a 0.22um microporous membrane, and freeze-dried to obtain the paclitaxel-loaded polymer micelles, which were diluted with water for injection or other solvents before use.
[0055] Dialysis method: Weigh 20mg of mPEG-PDLLA-CA block copolymer and 7mg of paclitaxel, dissolve them in 10mlL DMSO, sonicate for 10min, stir overnight to fully extend the chain segment, drop 20% distilled water in advance, stir for 2h, and use distilled water Dialysis was performed for 24 hours, and the dialysis medium was replaced at regular intervals. Finally, centrifuge at 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Critical micelle concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com