Process and apparatus for recovering lactide from polylactide or glycolide from polyglycolide
A technology of polylactide and lactide, which is applied in the field of recovering lactide from polylactide or recovering glycolide from polyglycolide and the device field, can solve the problem that it is difficult to economically realize large-scale technical processes, discontinuous And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
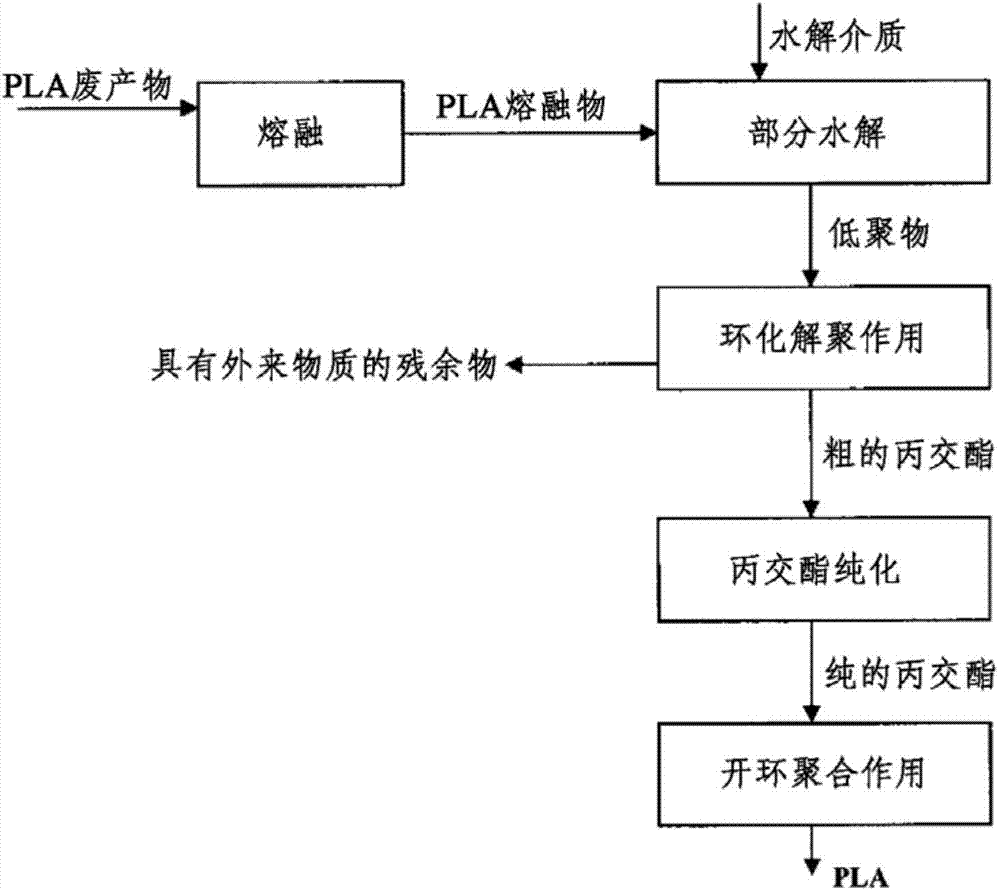
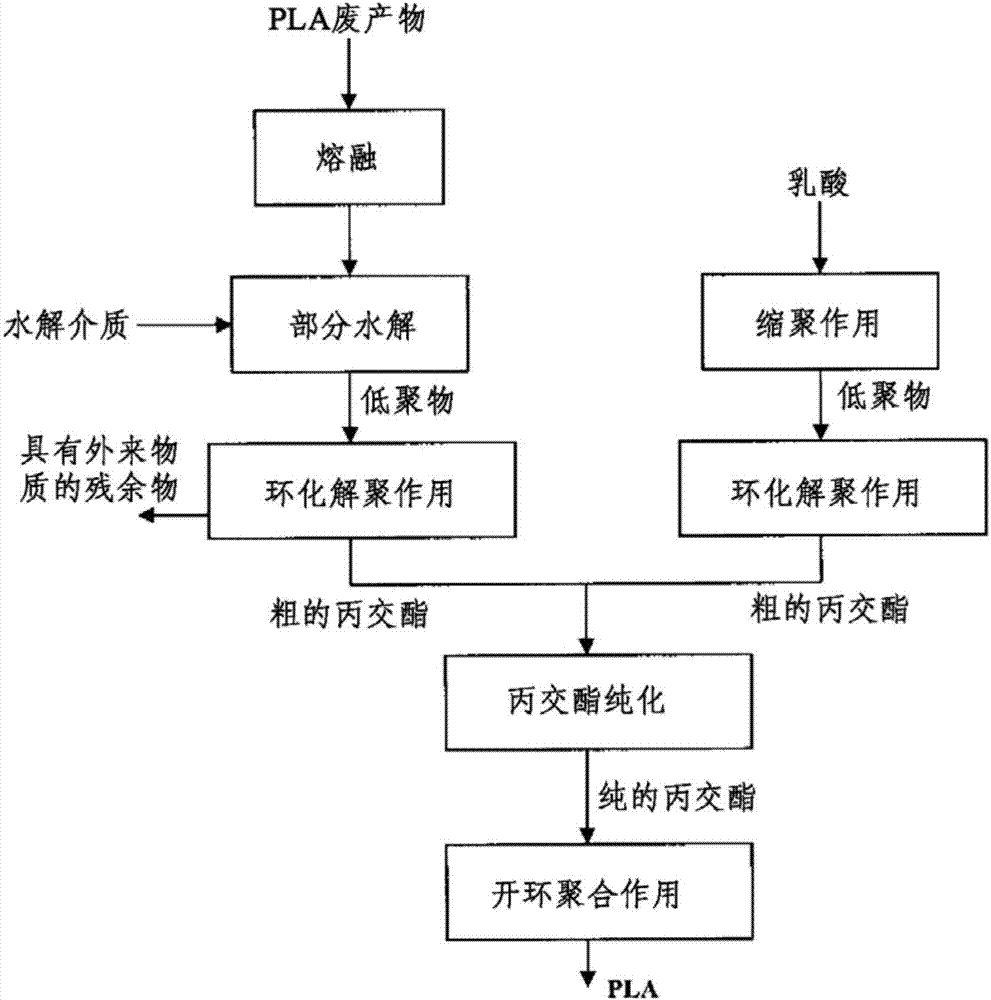
[0146] This example shows the basic applicability of the combination of cyclodepolymerization and hydrolysis for the conversion of PLA to crude lactide.
[0147] In a stainless steel laboratory pressure vessel lined with PTEE and closed, 30 g of PLA with an I.V of 1.80 dl / g (equivalent to a weight average molar mass of 230,000) was mixed with water in an amount of 0.70 g. The container was placed in a circulating air drying oven maintained at 190°C. After 6 hours, the autoclave was removed from the dry box and allowed to cool at room temperature. The container was opened and the resulting viscous material was removed and analyzed.
[0148] It was found by titration that the carboxyl end group concentration was 1250, corresponding mathematically to a molar mass of 800 Da.
[0149] In a three-necked glass flask, 20 g of the viscous substance was mixed with 0.184 mg of tin octoate with a stirrer and dissolved in toluene. After applying a vacuum of 5 mbar, the glass flask was w...
Embodiment 2
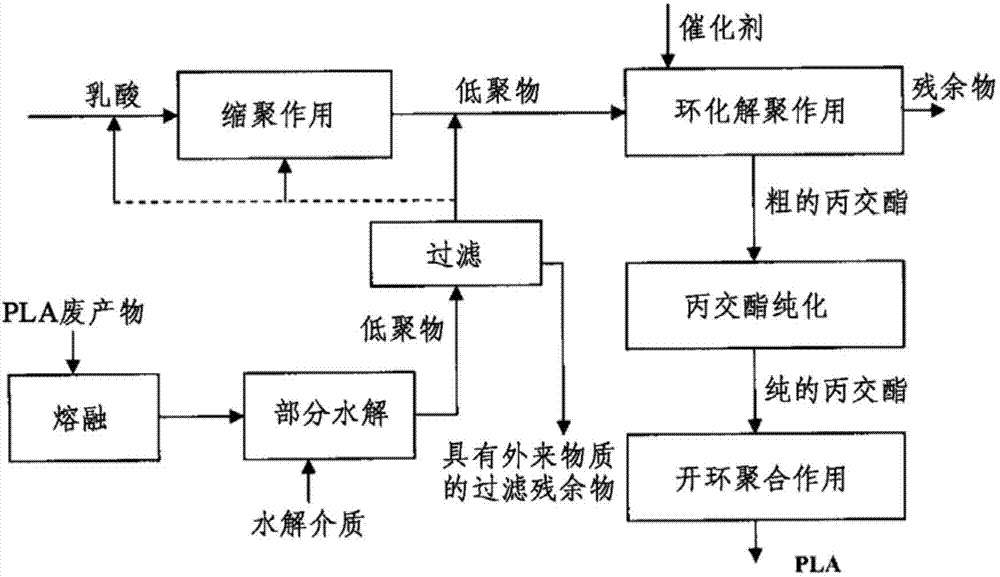
[0152] This example shows the effect of the method of the present invention. according to Figure 4 An extruder 1 was set up in the plant so that it took in and melted 1.5 kg / h of pulverized PLA waste product. The waste product showed an average I.V. of 1.64 dl / g (chloroform, 30°C).
[0153] The pressurization valve 8 at the end of the pipe section 2 is set to 30 bar. The melt temperature at the exit of the extruder was 210°C. By means of the metering pump 5 , 26.9 g / h of demineralized water are forced through the capillary 4 into the melt at the beginning of the pipe section. A sample of the melt after pressurization valve 8 showed a carboxyl end group concentration of 960 mmol / kg.
[0154] At the end of pipe section 2, metering pump 7 delivers 300 ppm Sn (as tin octoate) through capillary 6 to the hydrolyzed melt. In the depolymerization reactor 9, the melt temperature was set to 220°C. 70 g / h of liquid residue were removed from the reactor and 1,410 kg / h of crude lact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com