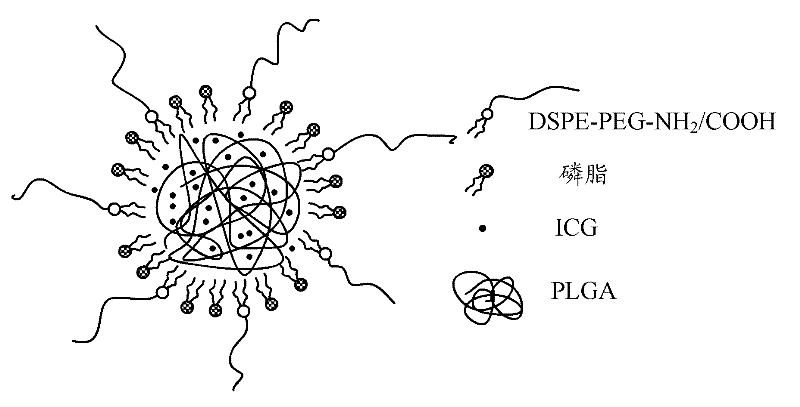
Fluorescence nanometer probe and preparation method thereof
A fluorescent nanoprobe and phospholipid technology, applied in the field of nanomedicine, can solve problems such as low stability and insufficient biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A method for preparing the above-mentioned fluorescent nanoprobes, comprising the steps of:
[0032] Step S1: provide ICG and PLGA, prepare ICG solution and PLGA solution respectively, and then mix ICG solution and PLGA solution to obtain a mixed solution of ICG and PLGA.
[0033] Among them, the ICG solution is preferably an aqueous solution of ICG with a concentration of 0.5-2 mg / mL. The PLGA solution is preferably a PLGA acetonitrile solution with a concentration of 1-5 mg / mL.
[0034] During the mixing process, measure the corresponding volume of ICG solution and PLGA solution according to the volume ratio of 1:5-20 and mix them.
[0035] Step S2: Provide phospholipids and DSPE-PEG-NH 2 (or DSPE-PEG-COOH), prepare phospholipids with DSPE-PEG-NH 2 (or DSPE-PEG-COOH) mixed solution. Specifically include the following steps:
[0036] Dissolving phospholipids in a mixed solvent of chloroform and methanol with a volume ratio of 8 to 10:1 to obtain a phospholipid sol...
Embodiment 1
[0044] (1), respectively prepare ICG aqueous solution with a concentration of 1 mg / mL and PLGA acetonitrile solution with a concentration of 2 mg / mL, and ultrasonically mix 100 μL of ICG aqueous solution and 1 mL of PLGA acetonitrile solution to obtain a mixed solution of ICG and PLGA;
[0045] (2) Weigh 0.24mg of soybean lecithin and dissolve it in a chloroform-methanol solvent with a volume ratio of 9:1 to obtain a phospholipid solution, then weigh 0.06mg of DSPE-PEG-COOH, add the phospholipid solution and DSPE-PEG-COOH into 3 mL of 4% ethanol aqueous solution, heated to 65°C and stirred for 3 min to obtain a mixed solution of phospholipids and DSPE-PEG-COOH;
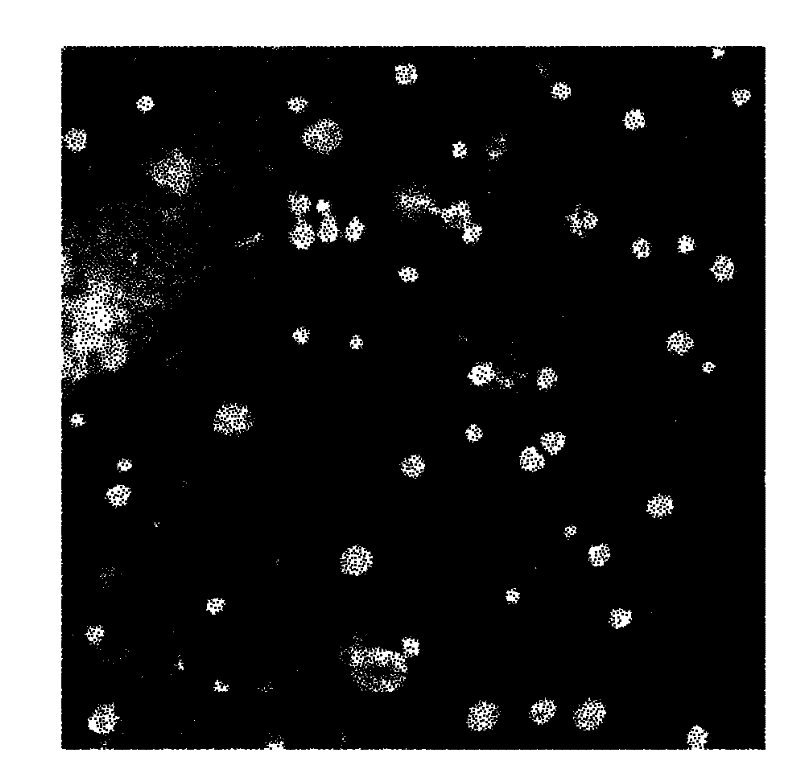
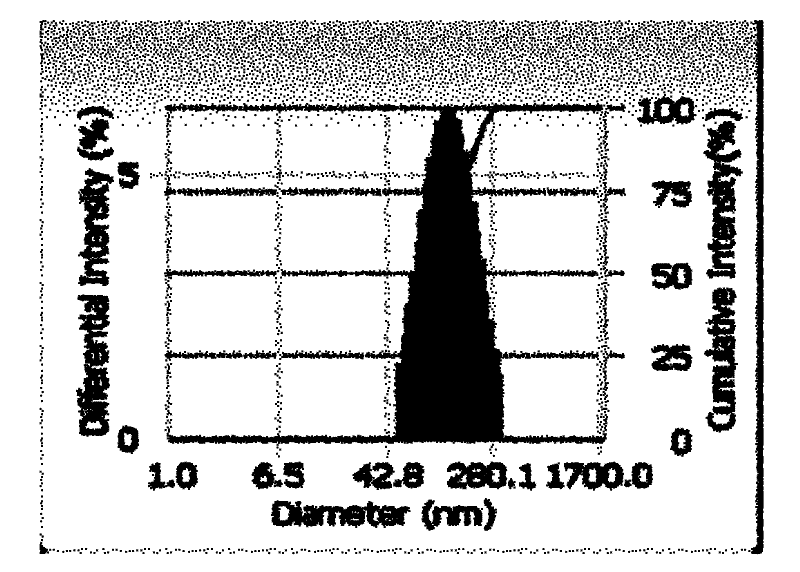
[0046] (3) Add the mixed solution of ICG and PLGA dropwise to the mixed solution of phospholipid and DSPE-PEG-COOH to react, and stir continuously at 35°C for 4h, during which the solvent is allowed to evaporate, and the fluorescent nanoprobe with an average diameter of 95.7nm is obtained. needle, such as figure 2 ,...
Embodiment 2
[0048] (1), respectively prepare an ICG aqueous solution with a concentration of 1 mg / mL and a PLGA acetonitrile solution with a concentration of 2 mg / mL, and ultrasonically mix 50 μL of the ICG aqueous solution with 1 mL of the PLGA acetonitrile solution to obtain a mixed solution of ICG and PLGA;
[0049] (2) Weigh 0.24mg of soybean lecithin and dissolve it in a chloroform-methanol solvent with a volume ratio of 9:1 to obtain a phospholipid solution, then weigh 0.06mg of DSPE-PEG-COOH, add the phospholipid solution and DSPE-PEG-COOH into 3 mL of 4% ethanol aqueous solution, heated to 65°C and stirred for 3 min to obtain a mixed solution of phospholipids and DSPE-PEG-COOH;
[0050] (3) Add the mixed solution of ICG and PLGA dropwise to the mixed solution of phospholipids and DSPE-PEG-COOH to react, stir continuously at 35°C for 4h, and allow the solvent to volatilize during this period to obtain fluorescent nanoprobes with an average diameter of 84.8nm Needle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com