Preparation method of (S)-oxiracetam
A technology of glycine ethyl ester hydrochloride and crude product, applied in the direction of organic chemistry, etc., can solve the problems of unattainable high purity, low purity, low preparation yield, etc., to reduce the amount of materials, reduce the content of impurities, and reduce pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
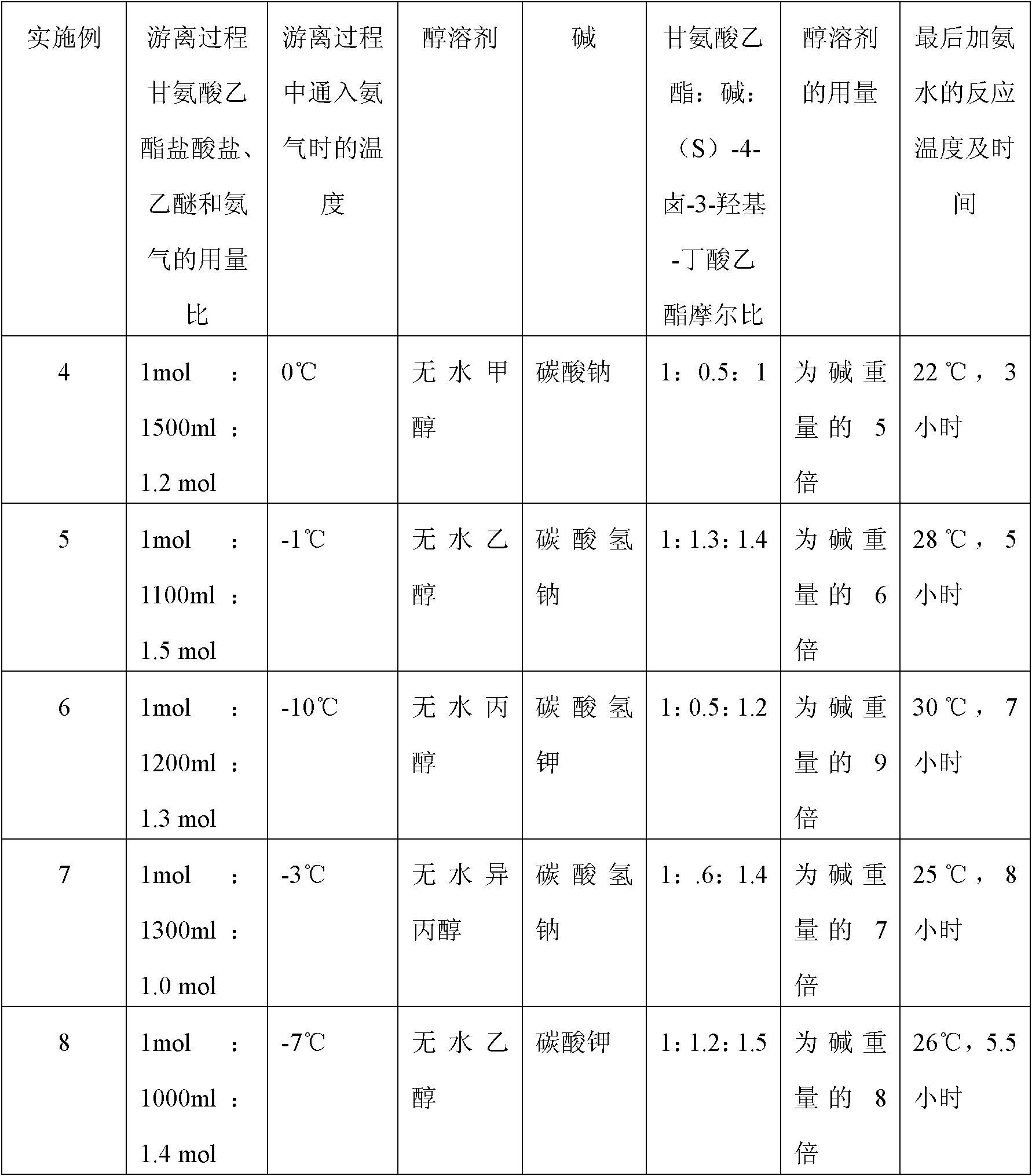
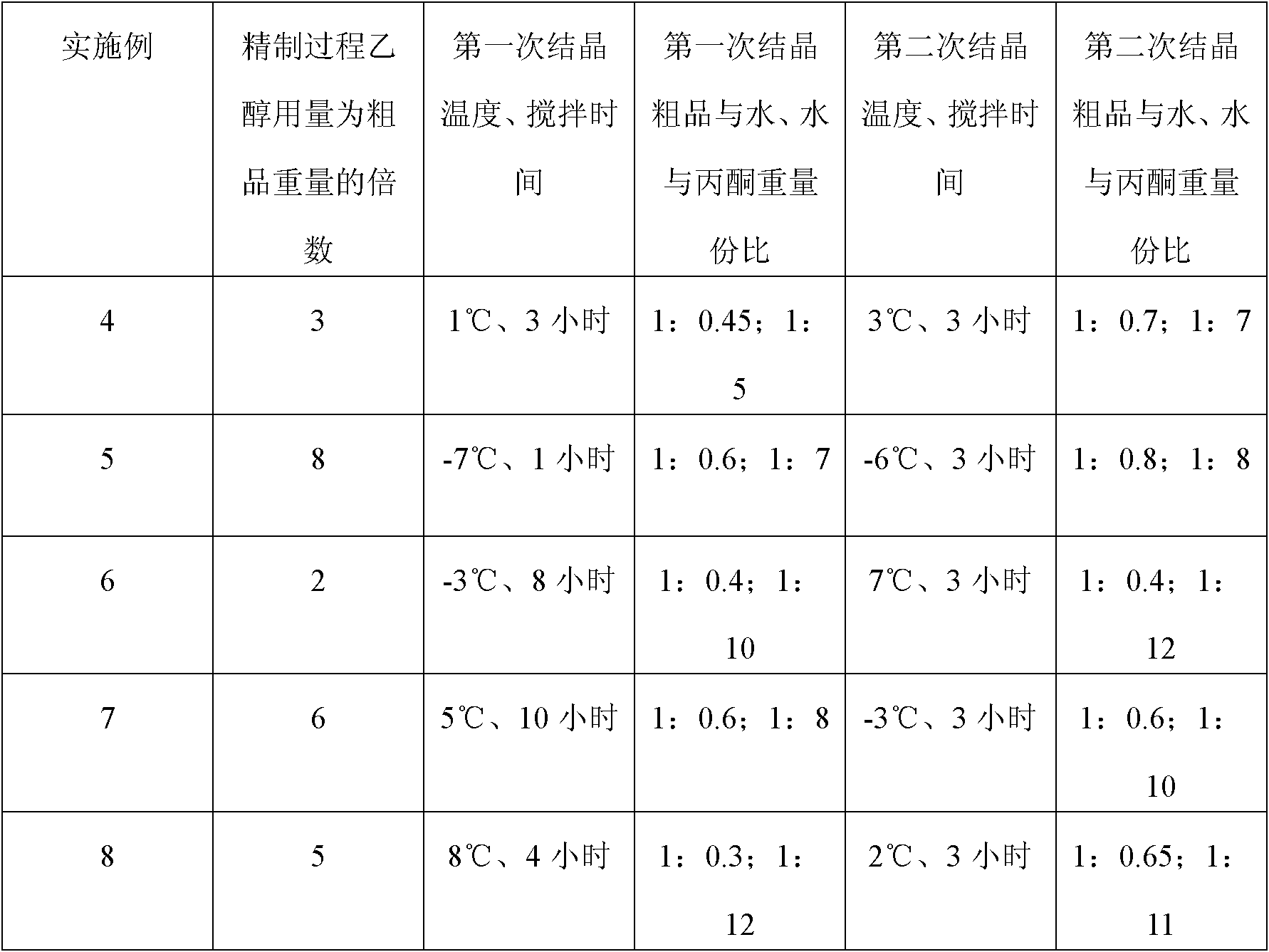
Embodiment 1
[0026] A kind of preparation method of (S)-oxiracetam, carry out as follows:
[0027] 1. Preparation of crude product:
[0028] (a) Add 139.6 g of glycine ethyl ester hydrochloride into 1200 ml of anhydrous ether, ice-cool to -2°C, and pass through 25.5 g of ammonia gas to fully dissociate glycine ethyl ester hydrochloride into glycine ethyl ester, wherein the glycine ethyl ester salt Salt: ether: ammonia=1mol: 1200ml: 1.5mol;
[0029] (b) Add 100.8g of sodium bicarbonate, 705ml of absolute ethanol and 250.0g of (S)-4-chloro-3-hydroxyl-butyric acid ethyl ester dropwise to the above glycine ethyl ester, and the dropping time is 3 hours , reacted at pH 8.0 and temperature 68°C for 28 hours;
[0030] (c) filter, fully wash the filtrate with ethanol, concentrate, then add 4 times the weight of the filtrate in chloroform to extract, concentrate, and separate by column chromatography; finally add 25% concentrated ammonia water and react at 21°C for 8 hours to obtain (S) - Oxirace...
Embodiment 2
[0037] A kind of preparation method of (S)-oxiracetam, carry out as follows:
[0038] 1. Preparation of crude product:
[0039] (a) Add glycine ethyl ester hydrochloride to anhydrous ether, cool it to 0°C, and feed ammonia gas to fully dissociate glycine ethyl ester hydrochloride into glycine ethyl ester, wherein glycine ethyl ester hydrochloride: ether: ammonia Gas=1mol: 1000ml: 1mol;
[0040] (b) Add sodium carbonate, anhydrous methanol and dropwise (S)-4-bromo-3-hydroxyl-butyric acid ethyl ester in above-mentioned glycine ethyl ester, described dropping time is 2.5 hours, at pH8.0, Reaction at 70°C for 25 hours;
[0041] (c) Filtrate, fully wash the filtrate with ethanol, concentrate, then add ethyl acetate 5 times the weight of the filtrate to extract, concentrate, and separate by column chromatography; finally add concentrated ammonia water and react at 20°C for 5 hours to obtain (S) - Oxiracetam crude;
[0042] Wherein ethyl glycine: sodium carbonate: (S)-4-bromo-3-h...
Embodiment 3
[0048] A kind of preparation method of (S)-oxiracetam, carry out as follows:
[0049] 1. Preparation of crude product:
[0050] (a) ethyl glycine hydrochloride is dissociated into ethyl glycine using ether and ammonia;
[0051] (b) Add sodium carbonate, absolute ethanol and (S)-4-iodo-3-hydroxyl-butyric acid ethyl ester dropwise to the above-mentioned glycine ethyl ester, the adding time is 2.3 hours, at pH8.5, Reaction at 67°C for 28 hours;
[0052] (c) Filtrate, fully wash the filtrate with ethanol, concentrate, then add dichloromethane 7 times the weight of the filtrate to extract, concentrate, and separate by column chromatography; finally add concentrated ammonia water and react at 30°C for 8 hours to obtain (S) - Oxiracetam crude;
[0053] Wherein ethyl glycine: sodium carbonate: (S)-4-iodo-3-hydroxyl-butyric acid ethyl ester=1: 0.5: 1.0, in mol ratio, the consumption of dehydrated alcohol is 8 times of sodium carbonate weight;
[0054] 2. Purification of crude produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com