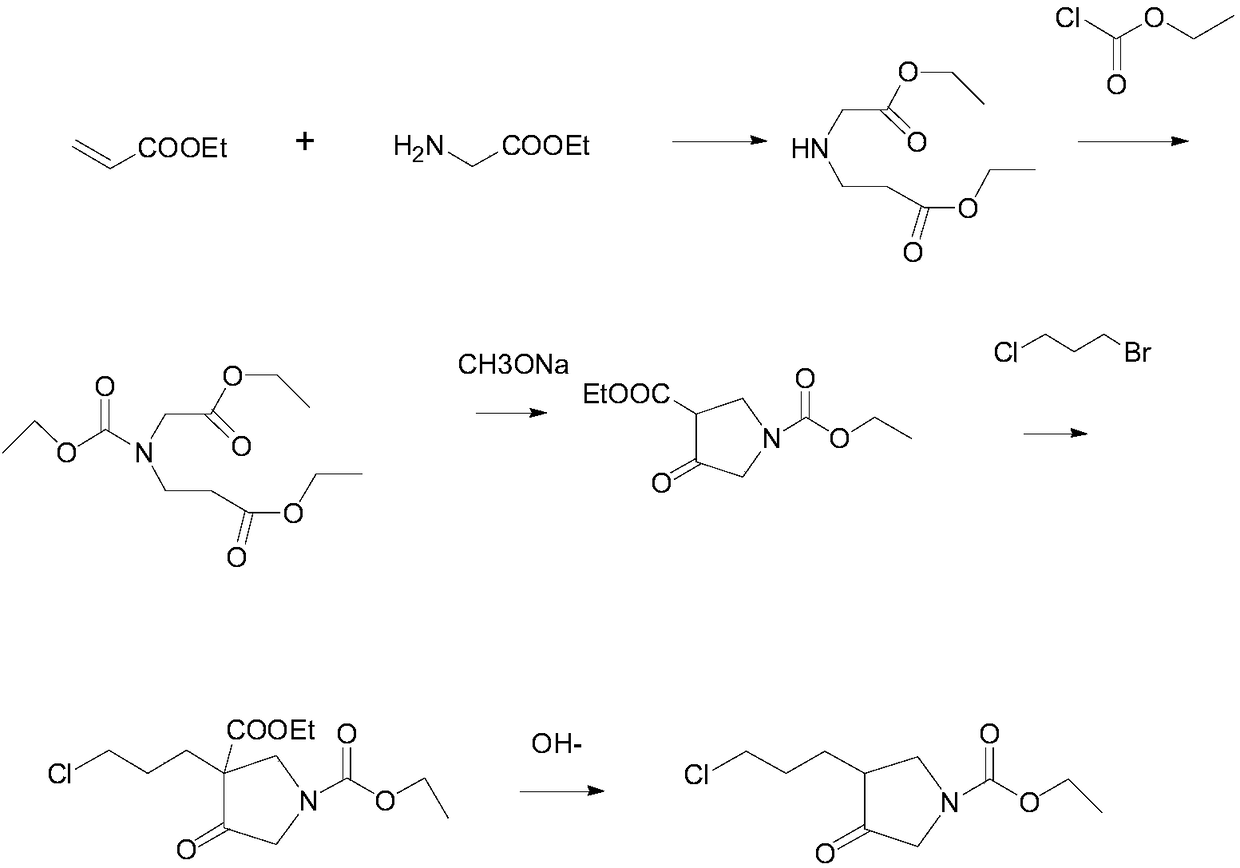
Method for preparing 3-(3-chloropropyl)-4-oxopyrrolidine-1-ethyl carboxylate
A technology of oxopyrrolidine and ethyl propionate, which is applied in the direction of organic chemistry, can solve the problems of high environmental hazards, complicated processes, and low safety, and achieve the effects of increased overall yield, safe reaction process, and simplified operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. At room temperature, add glycine ethyl ester (103.12g, 1mol), ethanol (458ml), and dropwise add ethyl acrylate (150g, 1.5mol) to the reactor, and the dropping time is controlled at about 5 hours (note that the temperature rise ), after dripping, keep it warm at 20±5°C for about 2 hours, and take a sample to control and detect that the reaction is complete. After the reaction, the system was transferred to a distillation reactor for distillation and concentrated to dryness at 45-50°C to obtain 197.3g of the product with a yield of 97.2%.
[0030] 2. At room temperature, add the previous step oil (197.3g, 0.972mol) to the reactor, add potassium iodide (4.32g, 26mmol), potassium carbonate (201.2g, 1.458mol), ethyl chloroformate (210.96g, 0.75mol), the temperature is 50±5°C, keep warm for 5 hours, filter with suction, rinse the filter cake with 100ml toluene, collect the filtrate, add sodium sulfate to the organic layer to dry, filter, rinse the filter cake with a small ...
Embodiment 2
[0035] 1. At room temperature, add glycine ethyl ester (103.12g, 1mol), ethanol (400ml), and dropwise add ethyl acrylate (105g, 1.05mol) to the reactor, and the dropping time is controlled at about 5 hours (note that the temperature rise ), after dripping, keep it warm at 20±5°C for about 2 hours, and take a sample to control and detect that the reaction is complete. After the reaction, the system was transferred to a distillation reactor for distillation and concentrated to dryness at 45-50°C to obtain 197.7g of the product with a yield of 97.4%.
[0036]2. At room temperature, add the previous step oil (197.7g, 0.974mol) to the reactor, add potassium iodide (2.71g, 16.3mmol), potassium carbonate (141.13g, 1.0227mol), ethyl chloroformate (158.5g , 1.461mol), the temperature is 50±5°C, keep warm for 5 hours, filter with suction, rinse the filter cake with 200ml of toluene, collect the filtrate, add sodium sulfate to the organic layer to dry, filter, rinse the filter cake with ...
Embodiment 3
[0041] 1. At room temperature, add glycine ethyl ester (103.12g, 1mol), ethanol (429ml), and dropwise add ethyl acrylate (130g, 1.3mol) to the reactor, and the dropping time is controlled at about 5 hours (note that the temperature rise ), after dripping, keep it warm at 20±5°C for about 2 hours, and take a sample to control and detect that the reaction is complete. After the reaction, the system was transferred to a distillation reactor for distillation and concentrated to dryness at 45-50°C to obtain 197.5g of the product with a yield of 97.3%
[0042] 2. At room temperature, add the previous step oil (197.5g, 0.973mol) to the reactor, add potassium iodide (1.4g, 21.2mmol), potassium carbonate (174.55g, 1.2649mol), ethyl chloroformate (184.74g , 1.70mol), the temperature is 50±5°C, keep warm for 5 hours, filter with suction, rinse the filter cake with 100ml of toluene, collect the filtrate, add sodium sulfate to the organic layer to dry, filter, rinse the filter cake with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com