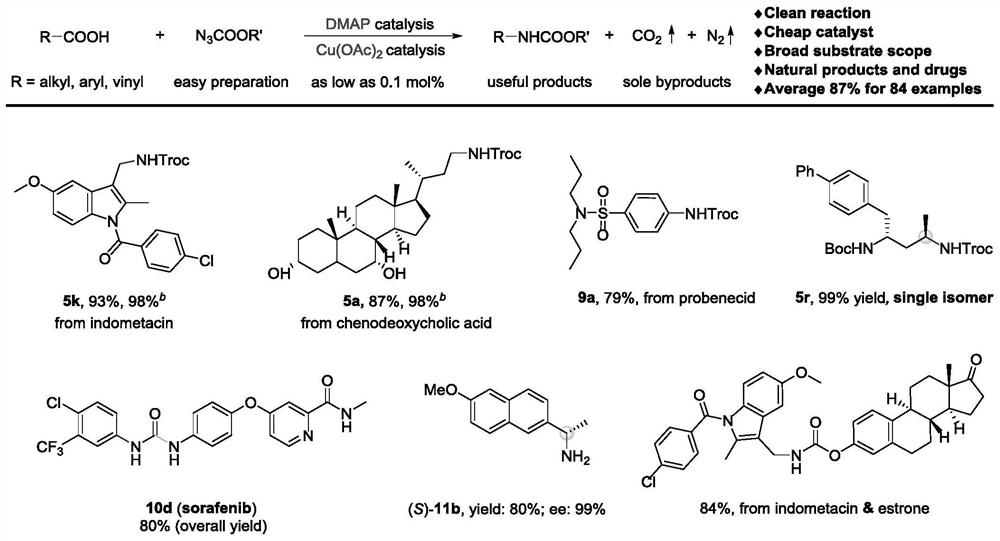
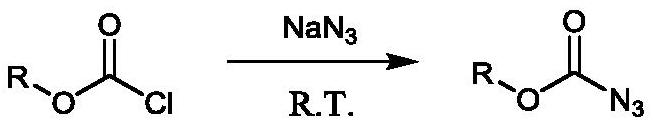Method for preparing amine compounds based on novel catalytic Curtius rearrangement reaction
An amine compound, rearrangement reaction technology, applied in the preparation of organic compounds, the preparation of aminohydroxy compounds, steroid compounds, etc., can solve the problem of few C-N bonds, and achieve the effect of efficient synthesis and rapid construction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of 2,2,2-trichloroethyl-((1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-)methyl)carbamate ester
[0053] The target product passes through the reaction process and post-processing. Purification by column chromatography afforded 94.0 mg of the target product (93% isolated yield).
[0054] 1 H NMR (400MHz, Chloroform-d) δ7.64(d, J=8.5Hz, 2H), 7.49(d, J=8.4Hz, 2H), 7.03(d, J=2.5Hz, 1H), 6.81(d ,J=9.0Hz,1H),6.66(dd,J=9.1,2.6Hz,1H),5.25(t,J=5.5Hz,1H),4.76(s,2H),4.53(d,J=5.5Hz ,2H),3.81(s,3H),2.42(s,3H); 13 C NMR (101MHz, Chloroform-d) δ168.4, 156.2, 154.7, 139.6, 136.5, 133.7, 131.3, 131.0, 129.9, 129.3, 115.6, 115.1, 112.1, 101.3, 95.7, 74.7, 55.8, 35.4, 13.2; )v 3344, 2929, 1732, 1682, 1591, 1478, 1221, 1045, 811, 721cm -1 ; HRMS (ESI) Calcd.for C21 h 18 Cl 4 N 2 o 4 Na[M+Na] + 524.9913,found 524.9908.
Embodiment 2
[0056] Preparation of 2,2,2-trichloroethyl((R)-3-((3R,7R,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl Hexadecylhydro-1H-cyclopenta[a]phenanthrene-17-)butyl)carbamate
[0057]
[0058] The target product passes through the reaction process and post-processing. Purification by column chromatography afforded 93.2 mg of the target product (87% isolated yield).
[0059] 1 H NMR (400MHz, Chloroform-d) δ4.95(t, J=6.1Hz, 1H), 4.72(s, 2H), 3.84(q, J=3.0Hz, 1H), 3.49–3.42(m, 1H) ,3.34–3.26(m,1H),3.21–3.12(m,1H),2.19(q,J=12.7Hz,1H),2.00–1.94(m,2H),1.92–1.86(m,1H),1.84 –1.78(m,2H),1.72–1.59(m,4H),1.52–1.45(m,6H),1.41–1.33(m,3H),1.31–1.09(m,7H),0.97(d,J= 6.6Hz,4H),0.90(s,3H),0.65(s,3H); 13 C NMR (101MHz, Chloroform-d) δ154.6, 95.9, 74.6, 72.1, 68.6, 56.1, 50.6, 42.9, 41.6, 40.0, 39.7, 39.5, 39.0, 36.0, 35.4, 35.2, 34.8, 33.9, 32.9, 30.8 ,28.5,23.8,22.9,20.7,18.7,11.9; IR (neat) v3445,3347,2932,2867,2133,1715,1520,1251,1141,731cm -1 ; HRMS (ESI) Calcd.for C 26 h 42 Cl 3 ...
Embodiment 3
[0061] Preparation of 2,2,2-trichloroethyl(4-(N,N-dipropylsulfamoyl)phenyl)carbamate
[0062]
[0063] The target product passes through the reaction process and post-processing. Purification by column chromatography afforded 67.8 mg of the target product (79% isolated yield). 1 H NMR (400MHz, Chloroform-d) δ7.77 (d, J = 8.7Hz, 2H), 7.57 (d, J = 8.7Hz, 2H), 7.29 (brs, 1H), 4.84 (s, 2H), 3.08 –3.04(m,4H),1.59–1.50(m,4H),0.86(t,J=7.4Hz,6H); 13 C NMR (101MHz, Chloroform-d) δ151.4, 140.9, 135.3, 128.6, 118.6, 95.1, 74.8, 50.2, 22.1, 11.3; IR (neat) v3319, 2966, 1751, 1596, 1533, 1207, 1151, 590cm -1 ; HRMS (ESI) Calcd.for C 15 h 22 Cl 3 N 2 o 4 S[M+H] + 431.0361,found 431.0362.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com