Hydroxyl compound terminal modified functional group and method for modifying hydroxyl compound by using hydroxyl compound terminal modified functional group
A hydroxyl compound and terminal modification technology, applied in the field of polymer materials, can solve the problems of product stability and performance, storage cycle impact, high pKa of preparation process, lack of unified and effective means, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
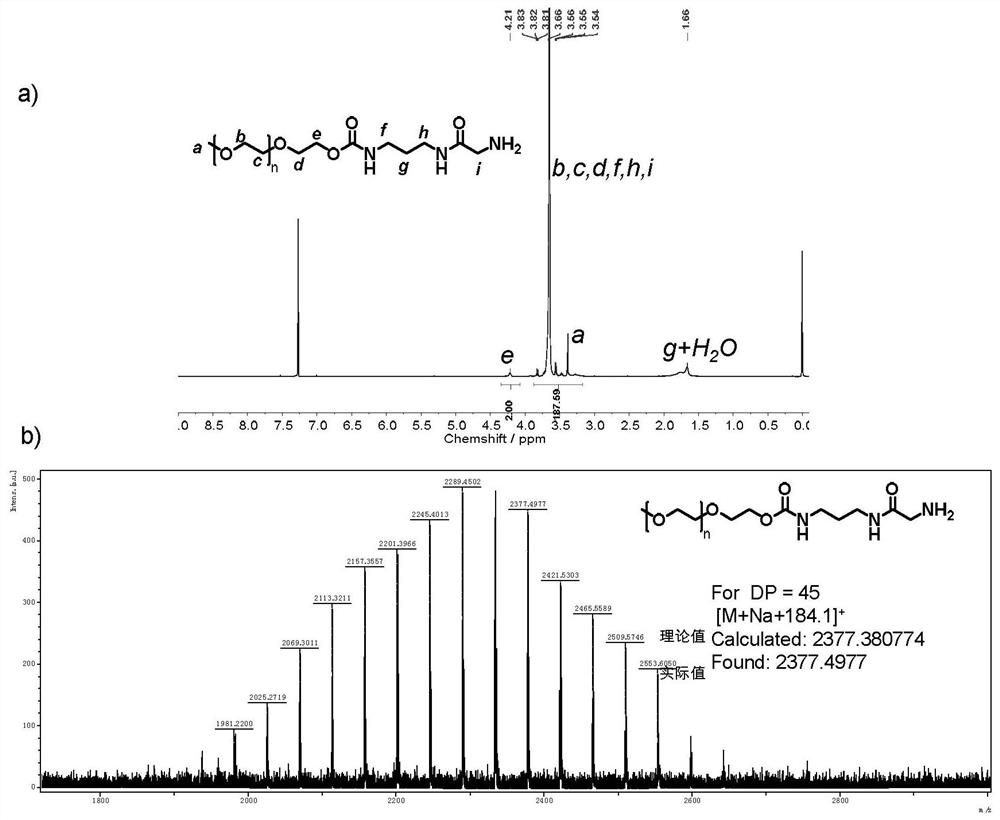
[0069] Example 1: Preparation method of the acid benzymonyl moisture molecule of benzyloxycarbonyl-protected.
[0070]
[0071] Compound 1 (5 g, 175.4 mmol) is dissolved in 100 ml of tetrahydrofuran, an ice bath, add anhydrous triethylamine (2.66 g, 263 mmol), adding azurizophosphate diphenyl ester (DPPA), an ice bath After stirring for 30 minutes, it was stirred at room temperature for 2 h. Carnation solvent, column chromatography (5 / 1, volume ratio) of petroleum ether / ethyl acetate, to obtain product 2 (3.5 g, yield 65%).
Embodiment 2
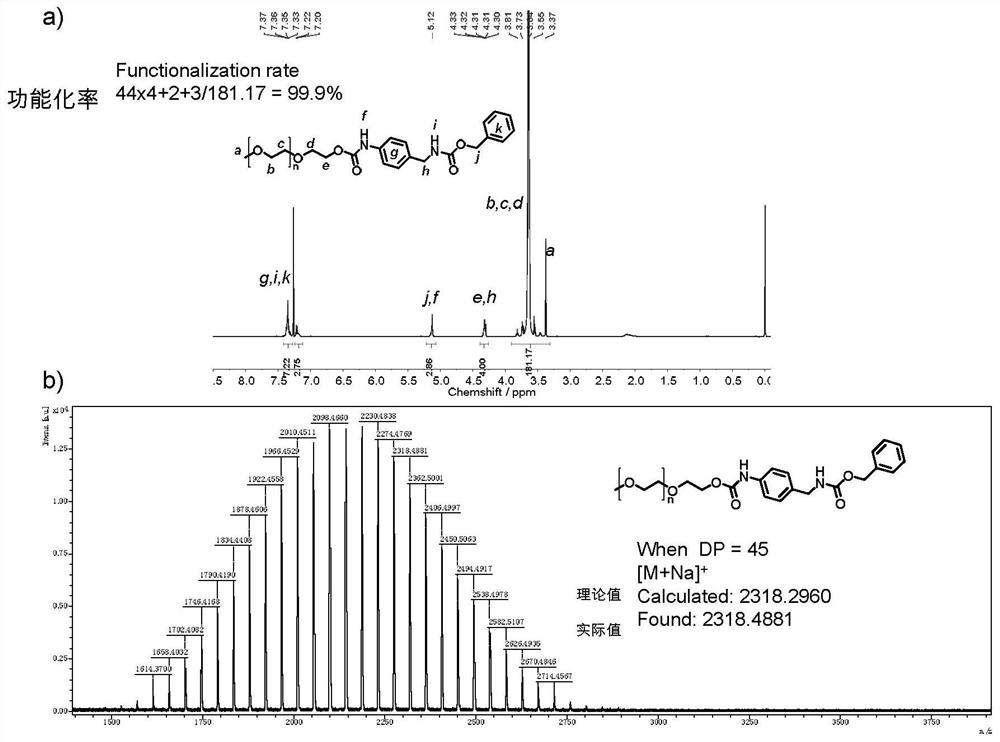
[0072] Example 2: a method of functionalized benzoxyl oxycarbonyl-protected benzylamine in a hydroxyl terminal having a polyethylene glycol monomethyl ether (n of approximately 45) having a molecular weight of 2000.
[0073]
[0074] Polyethylene glycol monomethyl ether (2.0 g, 1 mmol), benzyl oxycarbonyl-protected benzyl oxycarbonyl, and dibutyltin (DBTL) (40 μl) (40 μl) (40 μl) (40 μl) Unequarabone (100 mL), azeotrophone, 30 ml of non-toluene, and at 85 ° C for 6 h. At the end of the reaction, concentrated toluene, 10 ml of tetrahydrofuran dissolved, precipitated into 100 mL of anhydrous ether, repeatedly dissolved - precipitate 3 times, dried to give a white powder (2.10 g, yield, yield 91.0%).
Embodiment 3
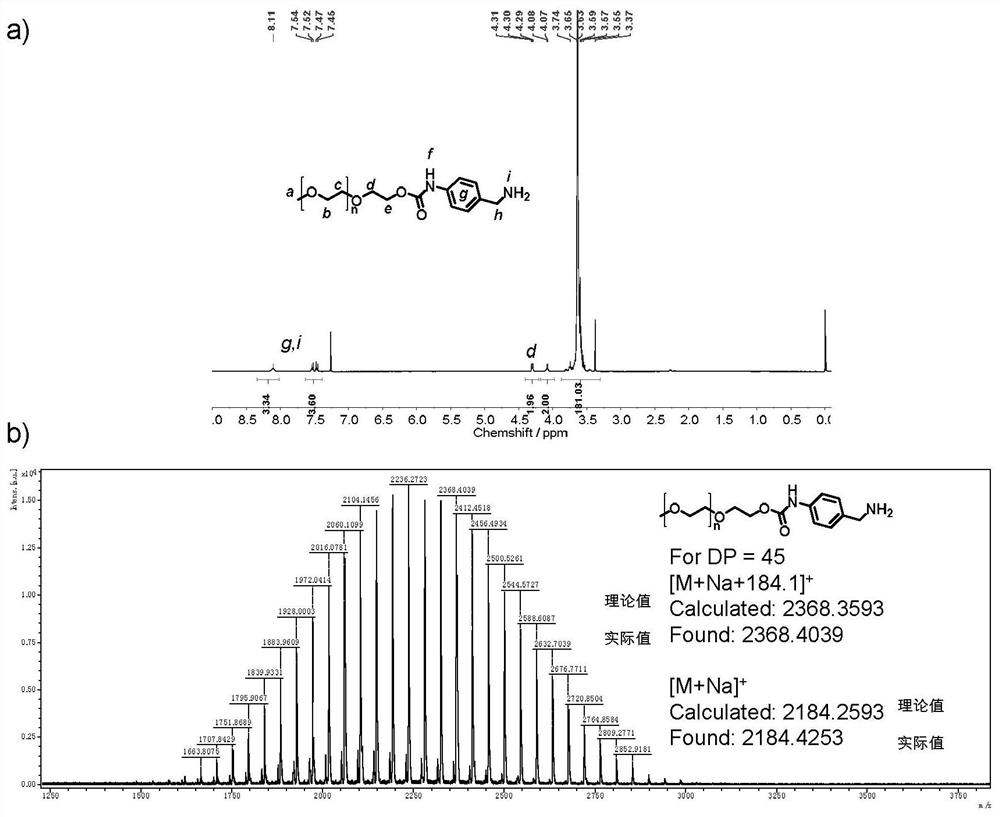
[0075] Example 3: a method of functioning benzylamine on a hydroxyl terminal having a polyethylene glycol monomethyl ether (n of approximately 45) having a molecular weight of 2000.
[0076]
[0077] The final product (2.0 g, 0.9 mmol) in Example 2 (2.0 g, 0.9 mmol) was added to 10% PD / C (0.2 g), and 15 ml of toluene is an azeotropic water. 20 ml of anhydrous methanol, nitrogen protection was added, and then resembled as hydrogen, reacted at 25 ° C for 2 hours, and after the solution had a solution of 220 nm, the solvent had a white powder (1.66 g, yield 87%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com