A kind of method for synthesizing multi-substituted pyrimidine derivatives by a two-step method
A technology of pyrimidine derivatives and multi-substitution, applied in the fields of organic synthesis and pharmaceutical intermediates, can solve the problems of low reaction yield and increased production cost, and achieve the effects of simple synthetic route, convenient operation and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
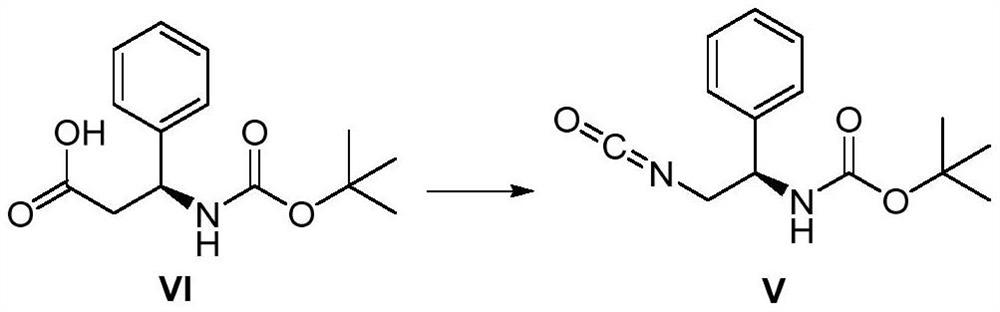
[0043] (1) the preparation of (R)-(2-isocyanato-1-phenylethyl) carbamate tert-butyl ester shown in formula V, its route is as follows:
[0044]
[0045] Operation steps: In a reactor, dissolve 2.5 g, 1.0 equiv. of N-Boc-beta-phenylalanine as shown in formula VI in 12 mL of tetrahydrofuran, and add 1.2 equiv. of chloroformic acid to it under ice bath conditions Ethyl ester and 2.4 equiv. triethylamine were kept in an ice bath, and the reaction was stirred for 1 h; then, under the ice bath condition, an aqueous solution of 3.0 equiv. of sodium azide was added to the reactor, and then the reaction was stirred at room temperature for 18 h. After the reaction, the reaction mixture was washed in a mixture of saturated brine and ethyl acetate, the organic phase was separated, dried with sodium sulfate and spin-dried to obtain 0.9 equiv. (R)-(2- tert-butyl isocyanato-1-phenylethyl)carbamate in 90% yield. Using high-resolution mass spectrometry (ESI+) detection, the found value was...
Embodiment 2
[0052] (1) the preparation of (R)-(2-isocyanato-1-phenylethyl) carbamate tert-butyl ester shown in formula V, its route is as follows:
[0053]
[0054] Operation steps: in the reactor, dissolve 2.5 g, 1.0 equiv. of N-Boc-beta-phenylalanine as shown in formula VI in 10 mL of methyl tert-butyl ether, and add to it under ice bath conditions. 0.9 equiv. ethyl chloroformate and 2 equiv. DBU, keep the ice bath, and stir the reaction for 1 h; then still under the ice bath condition, add 3.5 equiv. of sodium azide aqueous solution to the reactor, and then stir the reaction at room temperature for 18 h. After the reaction, the reaction mixture was washed in a mixture of dichloromethane and water, and the organic phase was separated, dried with sodium sulfate and spin-dried to obtain 0.75 equiv. (R)-(2-isocyanide shown in formula V) tert-butyl acid-1-phenylethyl)carbamate in 75% yield. Using high-resolution mass spectrometry (ESI+) detection, the found value was 295.1654.
[0055]...
Embodiment 3
[0061] (1) the preparation of (R)-(2-isocyanato-1-phenylethyl) carbamate tert-butyl ester shown in formula V, its route is as follows:
[0062]
[0063] Operation steps: In a reactor, dissolve 2.5 g, 1.0 equiv. of N-Boc-beta-phenylalanine as shown in formula VI in 15 mL of 2-methyltetrahydrofuran, and add 2.4 equiv. Phenyl chloroformate and 2.5 equiv. triethylamine were stirred and reacted for 1 h; then, an aqueous solution of 2.5 equiv. of sodium azide was added to the reactor, and then the reaction was stirred at room temperature for 18 h. After the reaction, the reaction mixture was washed in a mixture of dichloromethane and water, and the organic phase was separated, dried with sodium sulfate and spin-dried to obtain 0.81 equiv. (R)-(2-isocyanide as shown in formula V) tert-butyl acid-1-phenylethyl)carbamate in 81% yield. Using high-resolution mass spectrometry (ESI+) detection, the found value was 295.1652.
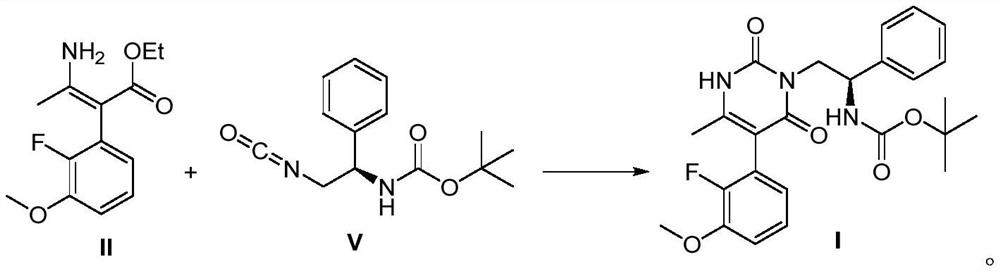
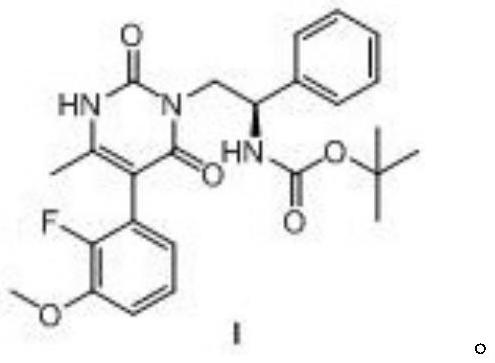
[0064] (2) the synthesis of the polysubstituted pyrimidine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com