Thioester peptide synthesis method
A technology of peptide synthesis and thioester, which is applied in the field of thioester peptide synthesis, can solve the problems of high synthesis cost of thioester peptide and limit the industrial production of thioester peptide, and achieve the effects of reducing synthesis cost, saving production time, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
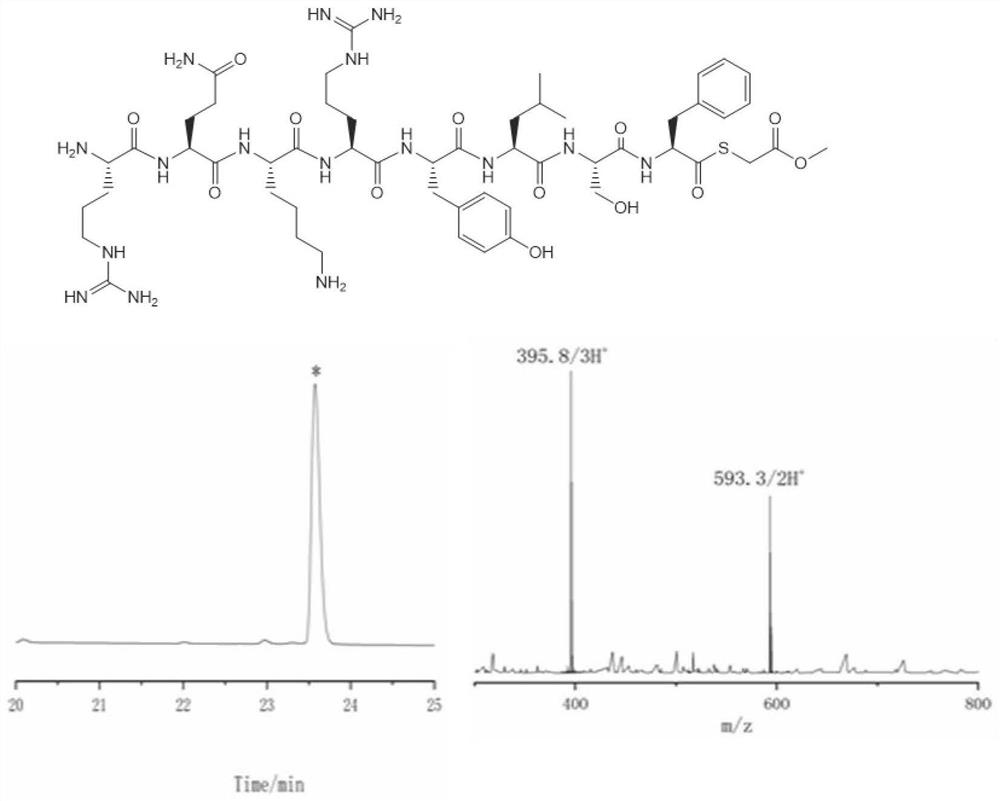
[0044] Example 1: Synthesis of thioester peptides with Phe at the C-terminus
[0045] The specific process of the preparation method for preparing thioester peptides in this embodiment includes the following steps:
[0046] (1) Preparation of polypeptide hydrazide whose C-terminus is Phe:
[0047] Preparation of 2-Cl(trt)-hydrazine resin: 5mL of DM containing 5% hydrazine hydrate (v / v) prepared in advance was added to 536mg of 2-Cl(trt)-Cl resin (loading 0.56mmol / g, 300μmol) , react at room temperature for 30 minutes, discard the reaction solution, and repeat once; add 5 mL of blocking reagent 5 mL of 20% (v / v) methanol in DMF solution, and wash the resin six times with DMF after 20 minutes of reaction.
[0048] Amino acid condensation: Weigh 697mg Fmoc-Phe-OH (6eq) and 255mg Oxyma (6eq) in a 10mL centrifuge tube, add 5.5mL of DMF and 280μL DIC (6eq) to dissolve the amino acid and add it to the solid-phase synthesis tube; in a metal bath ( After shaking at 55° C. for 40 minu...
Embodiment 2
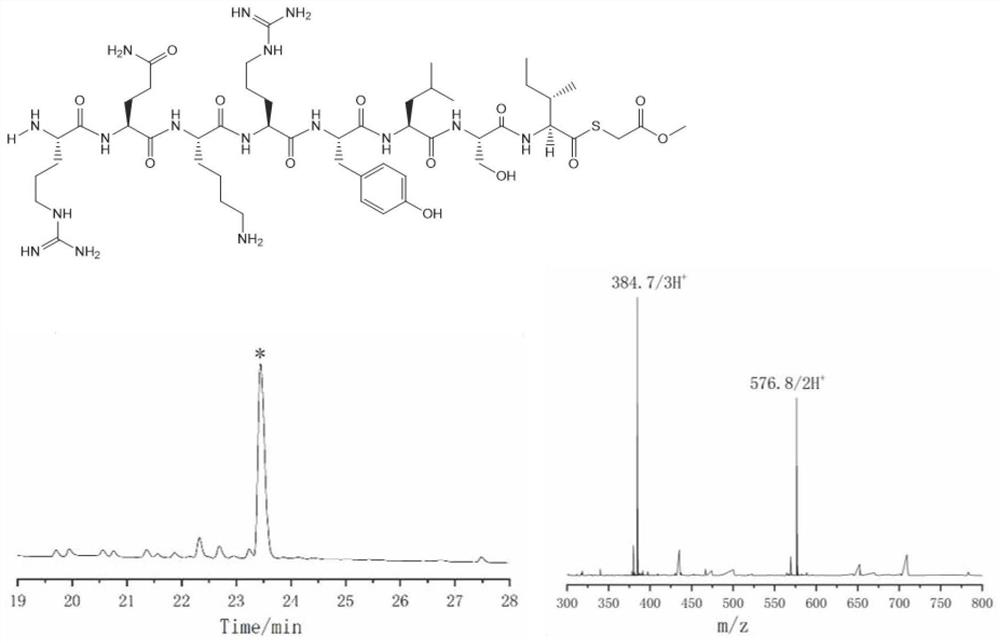
[0057] Embodiment 2: Synthesis of C-terminal thioester peptides of Ile
[0058] The specific process of the preparation method for preparing thioester peptides in this embodiment includes the following steps:
[0059] (1) Prepare the polypeptide hydrazide whose C-terminus is Ile:
[0060] Preparation of 2-Cl(trt)-hydrazine resin: 5mL of DM containing 5% hydrazine hydrate (v / v) prepared in advance was added to 536mg of 2-Cl(trt)-Cl resin (loading 0.56mmol / g, 300μmol) , react at room temperature for 30 minutes, discard the reaction solution, and repeat once; add 5 mL of 20% (v / v) methanol DMF solution and 5 ml of blocking reagent, and wash the resin with DMF six times after reacting for 20 minutes.
[0061] Amino acid condensation: Weigh 636mg Fmoc-Ile-OH (6eq) and 255mg Oxyma (6eq) in a 10mL centrifuge tube, add 5.5mL of DMF and 280μL DIC (6eq) to dissolve the amino acid and add it to the solid-phase synthesis tube; in a metal bath ( After shaking at 55° C. for 40 minutes, th...
Embodiment 3
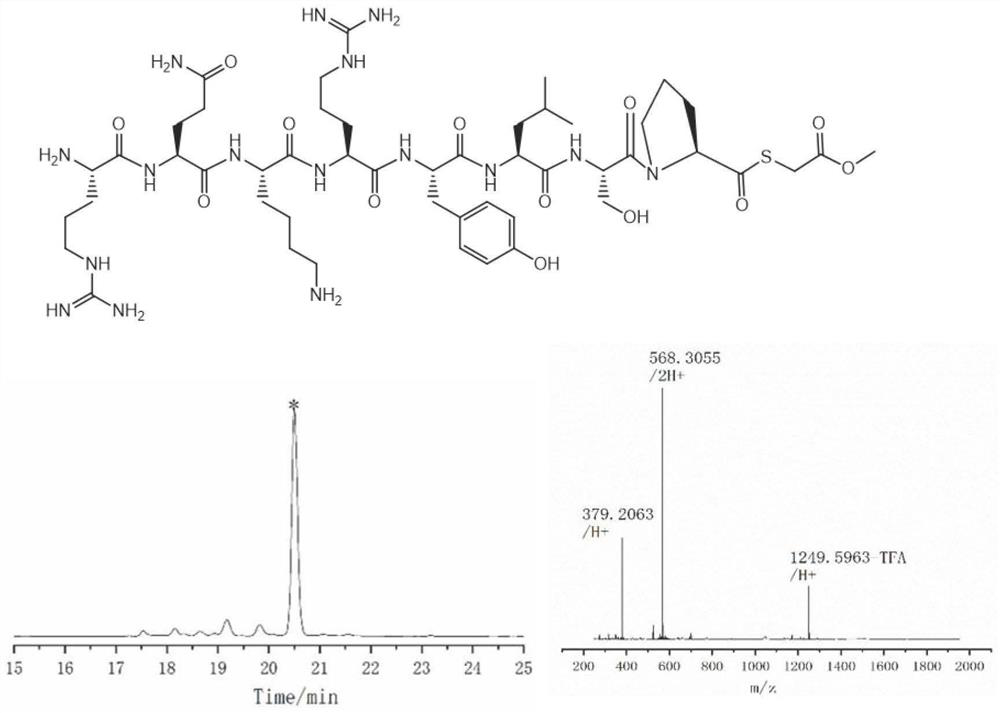
[0070] Example 3: Synthesis of thioester peptides with Pro at the C-terminus
[0071] The specific process of the preparation method for preparing thioester peptides in this embodiment includes the following steps:
[0072] (1) Preparation of polypeptide hydrazide whose C-terminus is Pro:
[0073] Preparation of 2-Cl(trt)-hydrazine resin: Add 5 mL of DM containing 5% hydrazine hydrate (v / v) prepared in advance to 536 mg of 2-Cl(trt)-Cl resin (loading 0.56 mmol / g, 300 μmol) , react at room temperature for 30 minutes, discard the reaction solution, and repeat once; add 5 mL of 20% (v / v) methanol DMF solution and 5 ml of blocking reagent, and wash the resin with DMF six times after reacting for 20 minutes.
[0074] Amino acid condensation: Weigh 606mg Fmoc-Pro-OH (6eq) and 255mg Oxyma (6eq) in a 10mL centrifuge tube, add 5.5mL of DMF and 280μL DIC (6eq) to dissolve the amino acid and add it to the solid-phase synthesis tube; in a metal bath ( After shaking at 55° C. for 40 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com