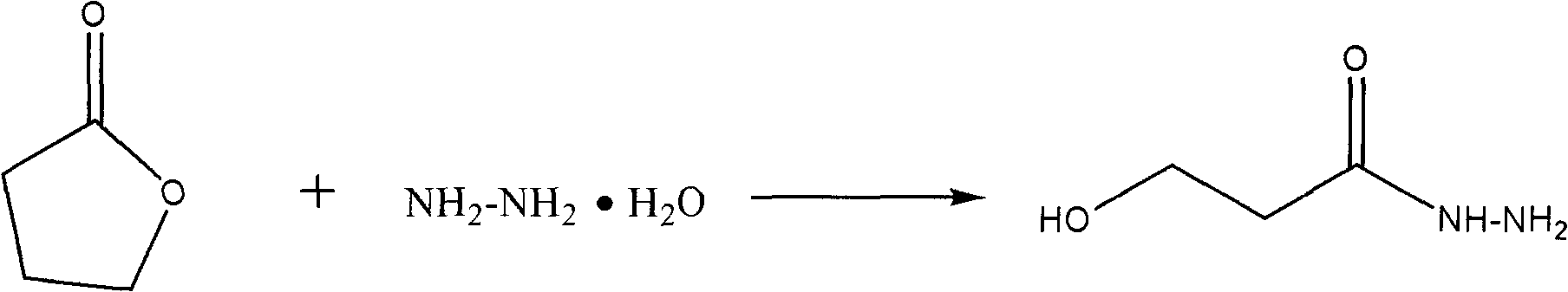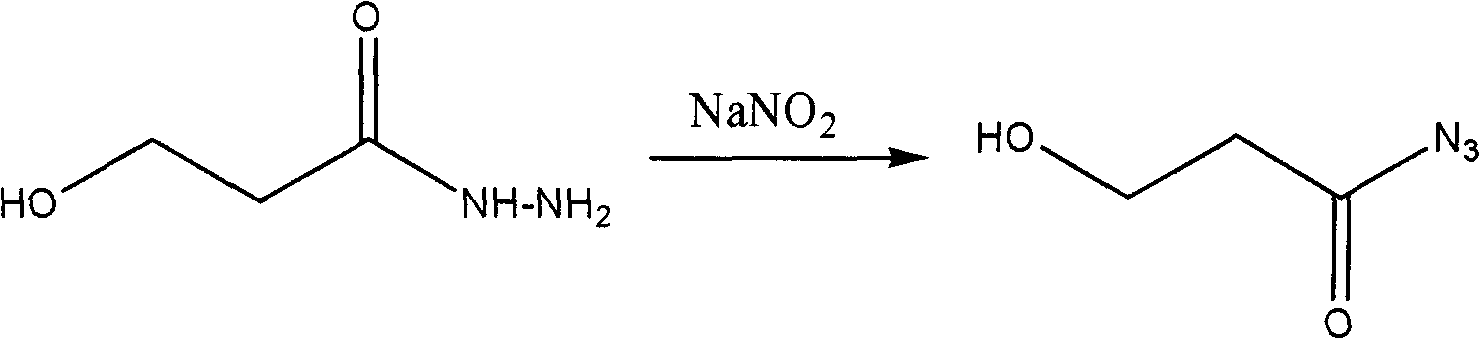Process for synthesizing an aminopropanol
A technology of aminopropanol and synthesis technology, applied in the field of synthesis technology of pharmaceutical intermediates, can solve the problems of only 12%, harsh reaction conditions, low yield and the like, and achieves the effects of easy operation and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] Processing step of the present invention is:
[0018] 1. Synthesis of Hydrazine
[0019] The reaction equation is:
[0020]
[0021] 2. Synthesis of Acyl Azides
[0022] The reaction equation is:
[0023]
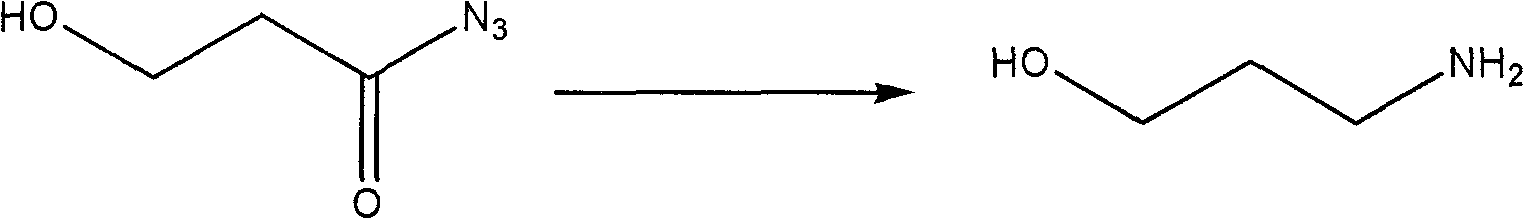
[0024] 3. Synthesis of 3-aminopropanol
[0025] The reaction equation is:
[0026]
[0027] When preparing acyl azide, the reaction temperature must be controlled below 0°C. When the temperature is too high, the acyl azide formed is unstable and will decompose immediately. At the same time, other by-products will be formed, and the yield of the target product will be very low.
[0028] When preparing acyl azide, the reaction is carried out under acidic conditions, so it should be washed with saturated sodium bicarbonate solution to keep the product neutral, then washed with distilled water, and finally dried with anhydrous sodium sulfate. The hydrochloric acid added to prepare the acyl azide is dilute hydrochloric acid, and the pH is not easy to be too ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com