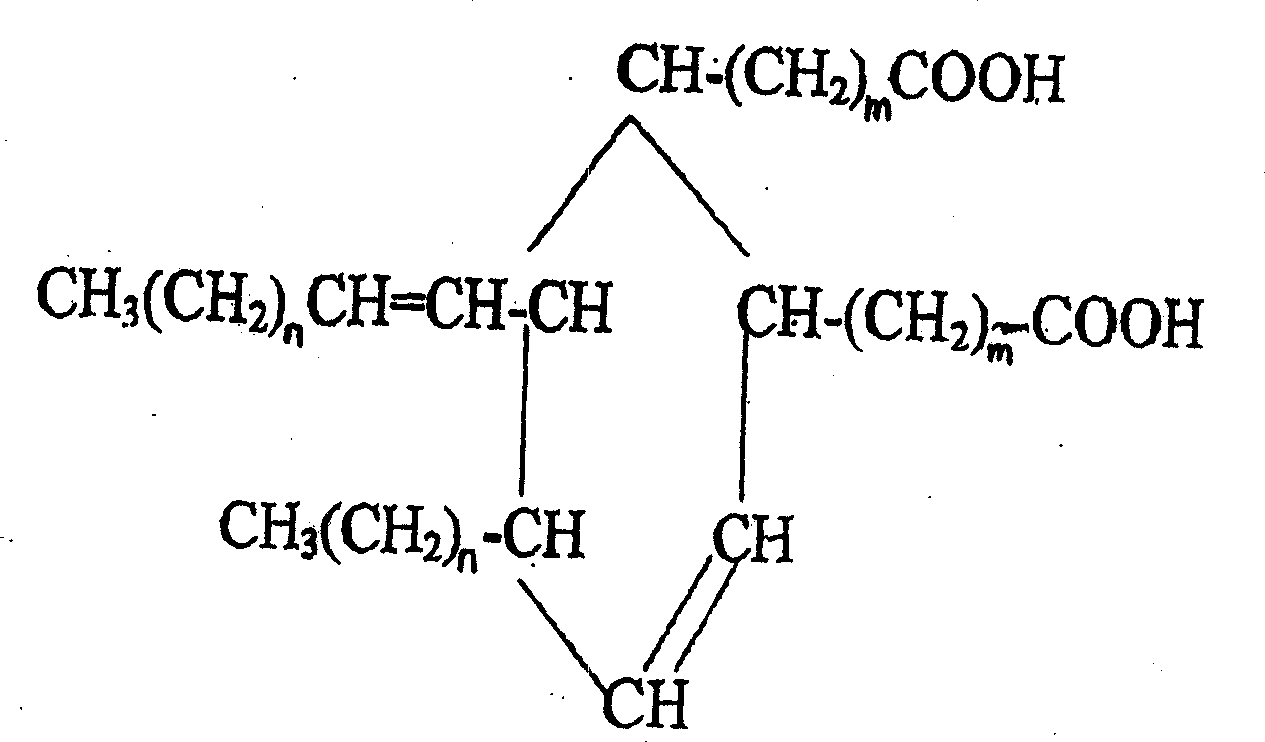
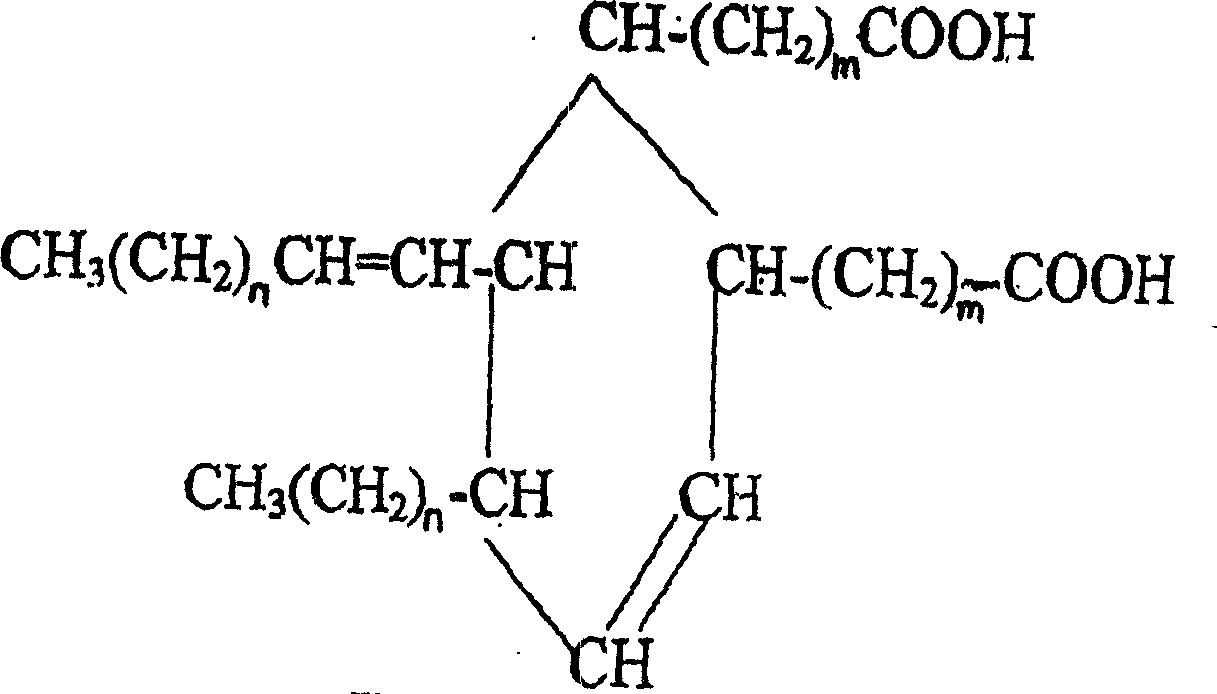
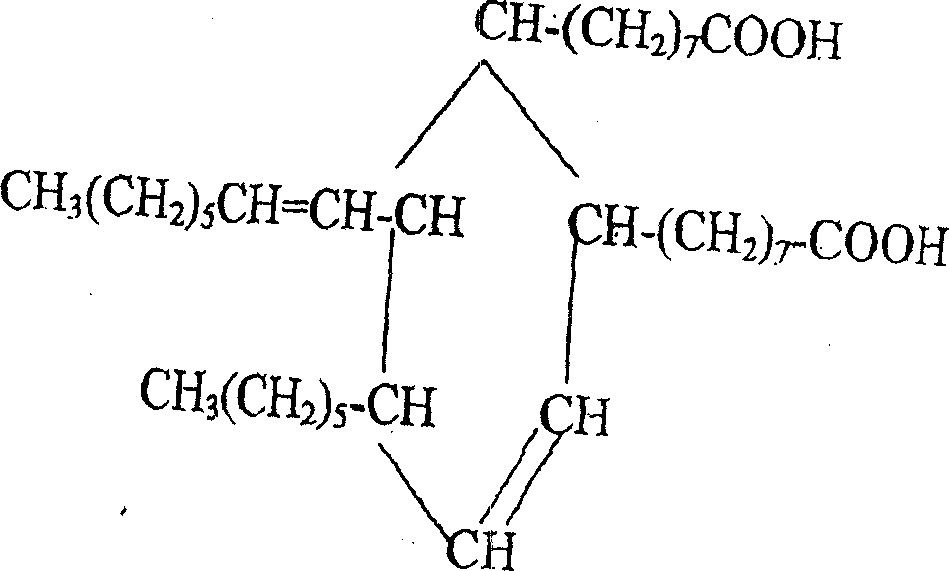
2-octyl-3,4-di(7-diisocyanateheptyl)-1-hexylcyclohexane and its preparation method and use
An isocyanate and dimer acid technology, applied in the preparation of carboxylic acid nitrogen-containing derivatives, organic chemistry and other directions, can solve the problems of difficult industrialization, low yield, high toxicity, etc., and achieve the advantages of favorable industrialization, simple operation, and mild conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1.2-octyl-3,4-bis(7-isocyanatoheptyl)-1-hexylcyclohexane)
[0037] step 1:
[0038] Put 350g xylene and 30g Pd (5%) / activated carbon catalyst (wet: dry weight 15g, 0.45g Pd) suspension into 2 liters of hydrogenation autoclave, then add 320g dimer acid (98%), draw separately Vacuum, nitrogen replacement, hydrogen replacement. The reaction mixture was stirred at 170-190° C., 1.5-2.0 Mp for about 7.5 hours, until the hydrogen absorption was complete, and the stirring was stopped. The material was filtered off under nitrogen pressure, leaving the catalyst in the filter. Use 350g xylene to backwash the catalyst into the reactor, add 320g dimer acid, repeat the hydrogenation, the catalyst activity does not decrease, carry out the hydrogenation 5 times in the same way, and reuse the catalyst 4 times, the obtained reaction mixture (colorless and transparent) was heated and dehydrated to obtain saturated dimer acid, which was reserved for the next step.
[0039] Step...
Embodiment 2
[0057] The preparation of embodiment 2.2-octyl-3,4-bis(7-isocyanatoheptyl)-1-hexylcyclohexane
[0058] According to the method similar to Example 1, only the reactions of steps 3 and 4 are combined. After adding sodium azide, the temperature is raised to above 120°C, and the azide compound generated by the sodium chloride generated without filtering is directly decomposed. Finish the product until the gas is no longer released, then filter sodium chloride, remove the solvent, and then molecularly distill under reduced pressure to obtain the target product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com