Anti-tumor long-circulating target liposomes for injections
A liposome-targeted, anti-tumor technology, applied in anti-tumor drugs, liposome delivery, medical preparations of non-active ingredients, etc., to achieve improved anti-tumor effects, facilitate industrialization, and efficiently kill tumor cells Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
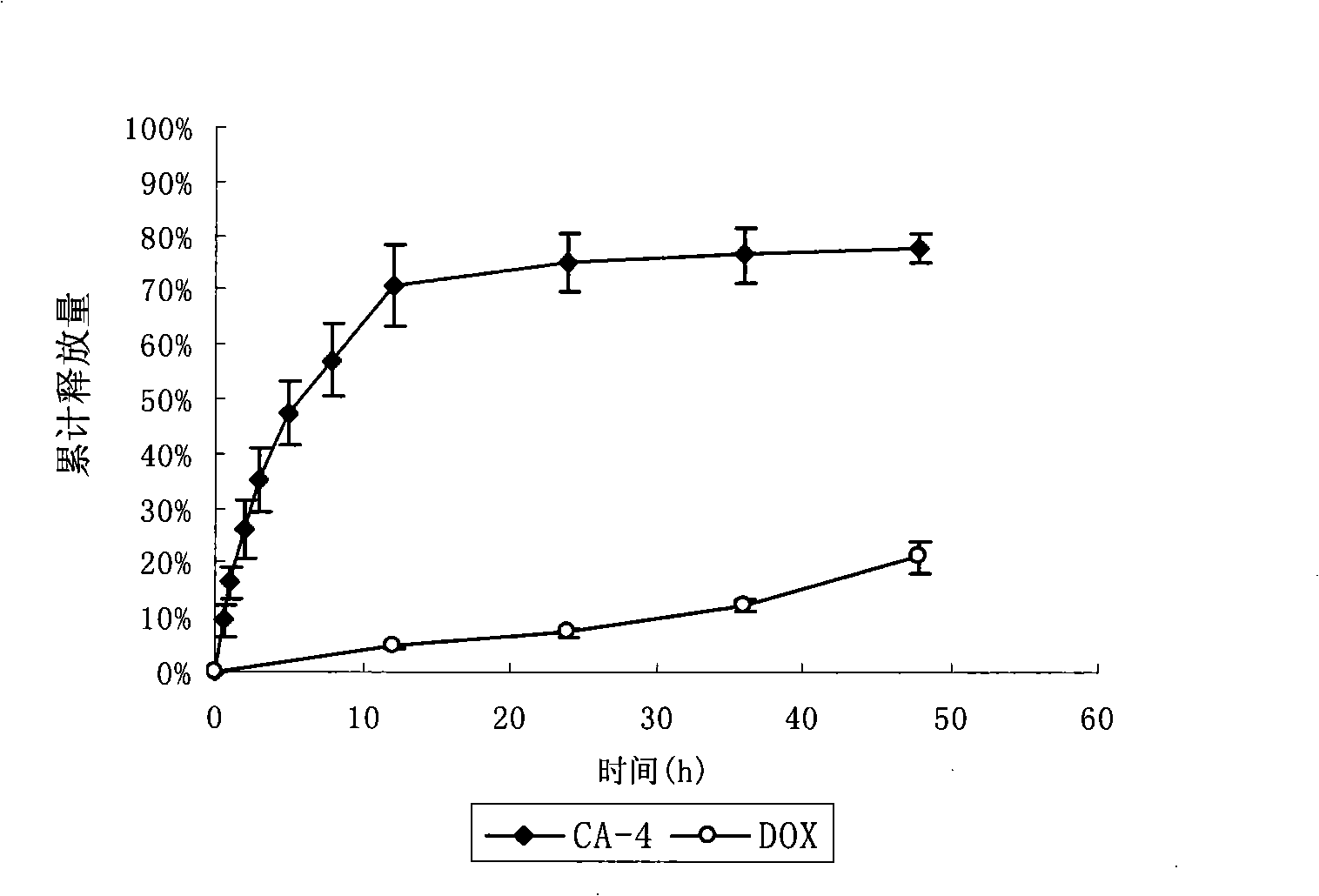
[0045] RGD-modified long-circulating liposome RGD-L [CD] simultaneously encapsulating CA-4 and adriamycin
[0046] The formulations of liposomes are: EPC:CHOL:DSPE-PEG:CA-4 (25:1.28:6.24:2) (unit: mg). Precisely weigh the prescribed amount of EPC, CHOL, DSPE-PEG, DSPE-PEG-RGD and CA-4 into a pear-shaped bottle, and add an appropriate amount of chloroform to dissolve it. 37 ℃ reduced pressure rotary evaporation into a uniform transparent film. Add 123 mM ammonium sulfate solution and sonicate in a water bath until blue opalescence appears. The 200nm polycarbonate film was extruded 5 times. Pass the prepared liposomes through a Sephadex G50 column, eluting with PBS buffer (pH 7.4) as the mobile phase, collect the liposomes, heat the collected liposomes in a 40℃ water bath, and add adriamycin Incubate the powder for 20 minutes and shake it from time to time to obtain this product.
Embodiment 2
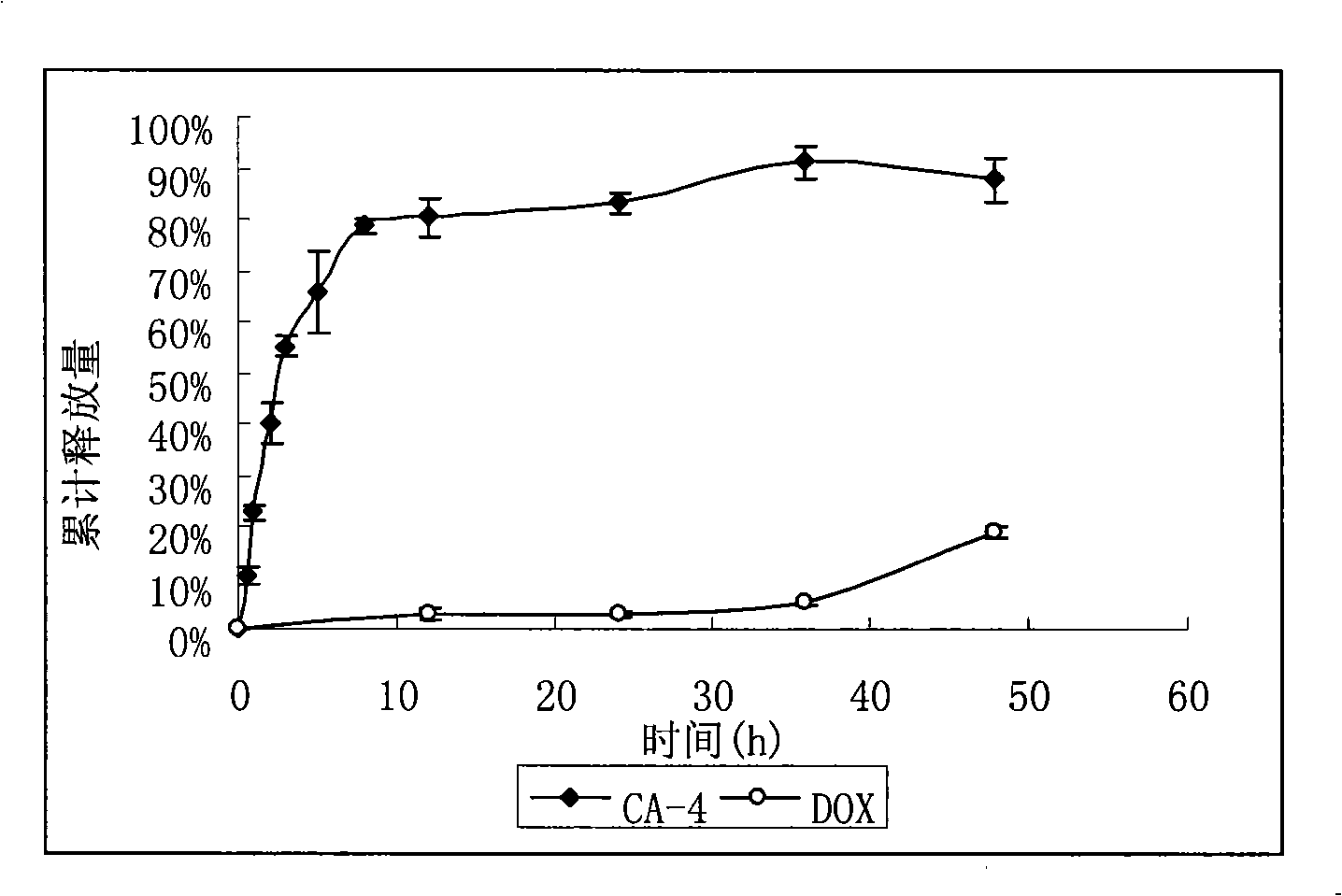
[0048] RGD-modified long-circulating liposome RGD-L [CD] simultaneously encapsulating CA-4 and adriamycin
[0049] The formulations of liposomes are HSPC:CHOL:DSPE-PEG:CA-4 (25:1.28:6.24:2) (unit: mg). Precisely weigh the prescribed amount of HSPC, CHOL, DSPE-PEG, DSPE-PEG-RGD and CA-4 into a pear-shaped bottle, and add an appropriate amount of chloroform to dissolve it. 37 ℃ reduced pressure rotary evaporation into a uniform transparent film. Add 123 mM ammonium sulfate solution and sonicate in a water bath until blue opalescence appears. The 200nm polycarbonate film was extruded 5 times. Pass the prepared liposomes through a Sephadex G50 column, eluting with PBS buffer (pH 7.4) as the mobile phase, collect the liposomes, heat the collected liposomes in a 40°C water bath, and add Amygdalin Incubate the vegetarian powder for 20 minutes and shake it from time to time to obtain this product.
Embodiment 3
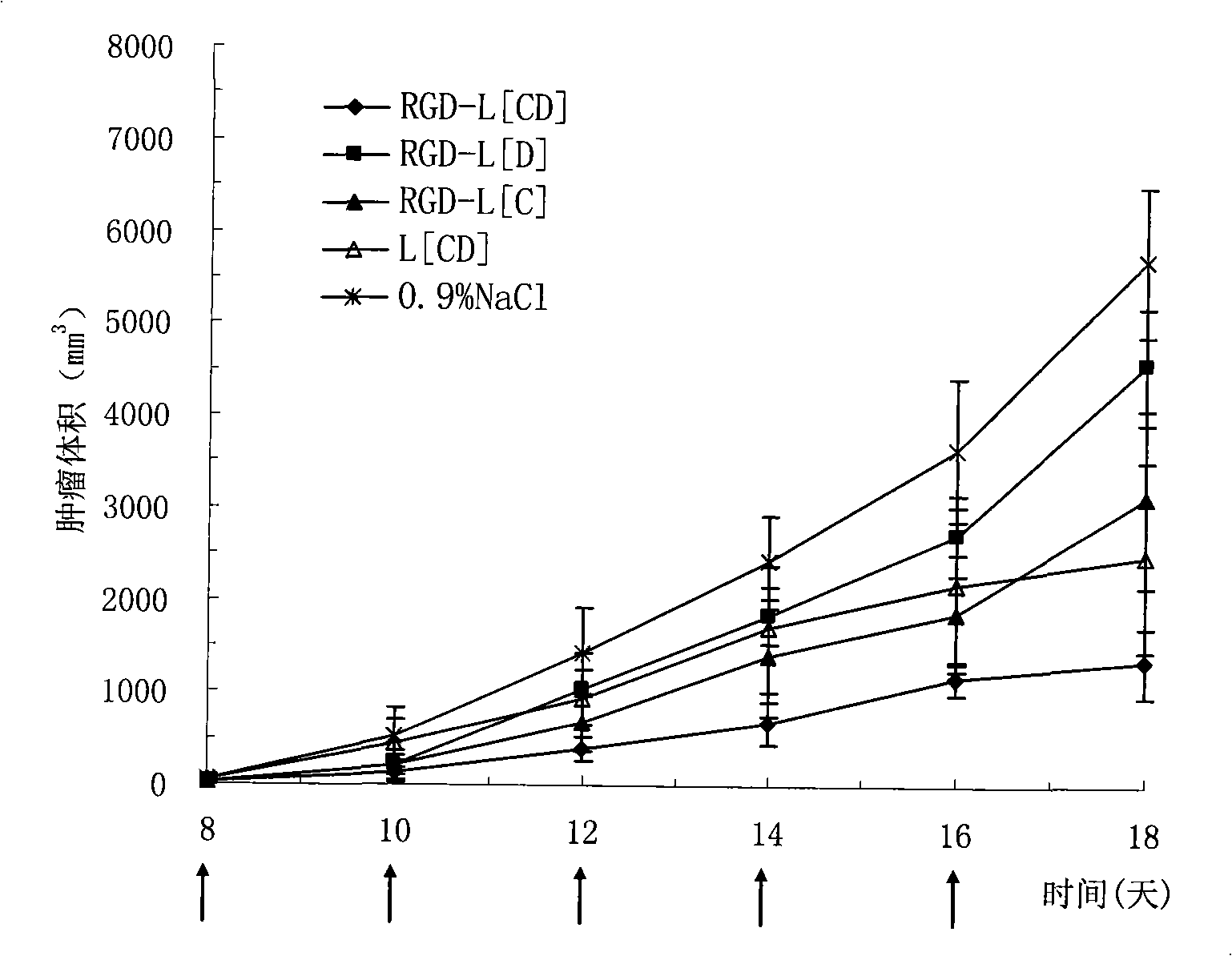
[0051] RGD-modified long-circulating liposome RGD-L [CD] simultaneously encapsulating CA-4 and adriamycin
[0052] The formulations of liposomes are: EPC:CHOL:DSPE-PEG:CA-4 (25:1.28:6.24:2) (unit: mg). Precisely weigh the prescribed amount of EPC, CHOL, DSPE-PEG, DSPE-PEG-RGD and CA-4 into a pear-shaped bottle, and add an appropriate amount of chloroform to dissolve it. 37 ℃ reduced pressure rotary evaporation into a uniform transparent film. Add 50 mM ammonium sulfate solution, and sonicate in a water bath until blue opalescence appears. The 200nm polycarbonate film was extruded 5 times. Pass the prepared liposomes through a Sephadex G50 column, eluting with PBS buffer (pH 7.4) as the mobile phase, collect the liposomes, heat the collected liposomes in a 37°C water bath, and add adriamycin Incubate the powder for 20 minutes and shake it from time to time to obtain this product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com