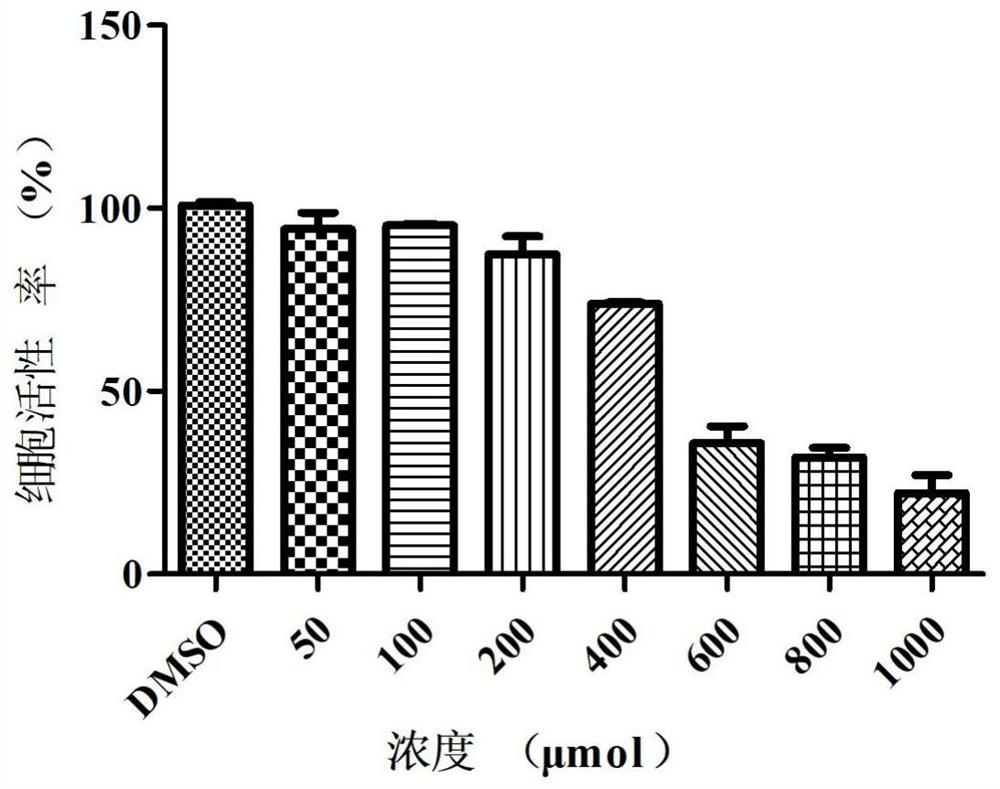
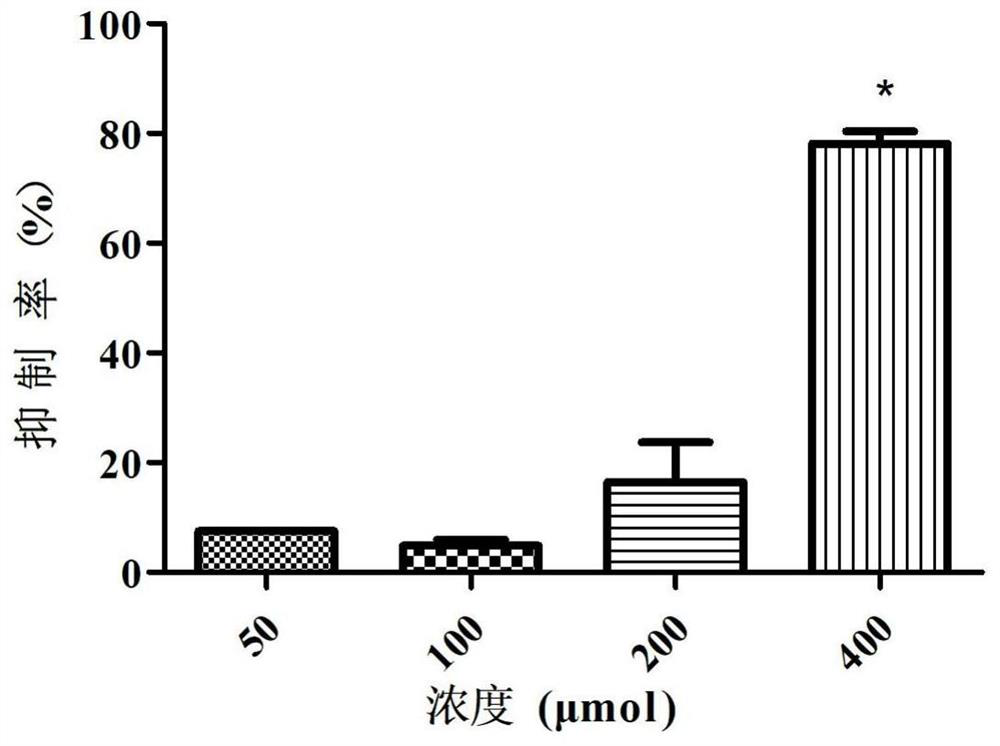
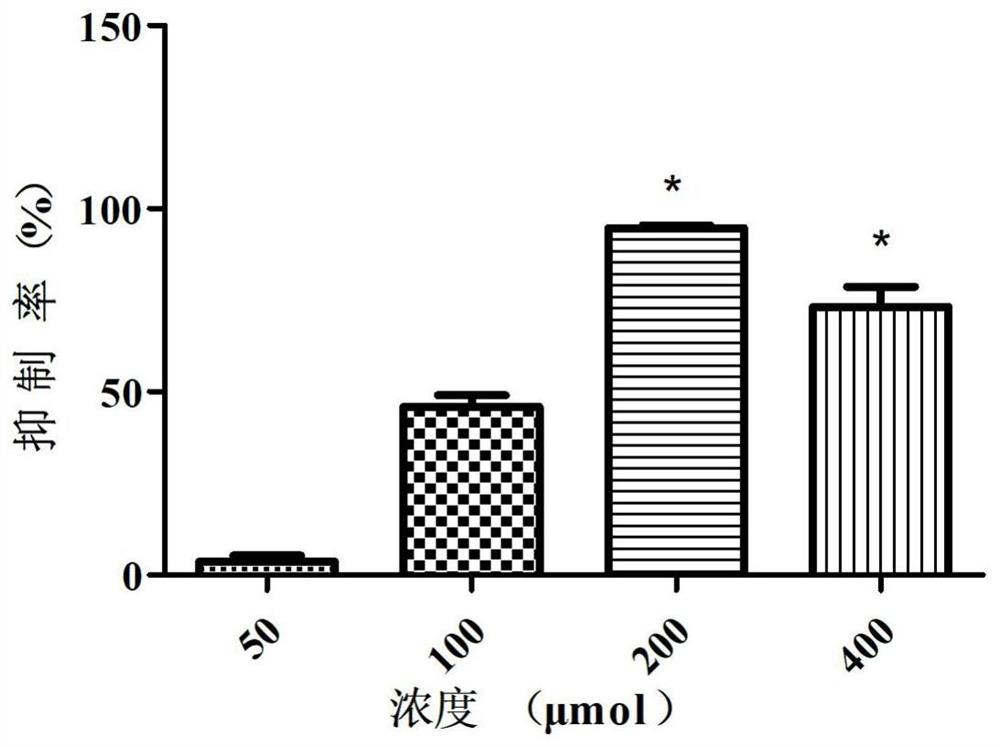
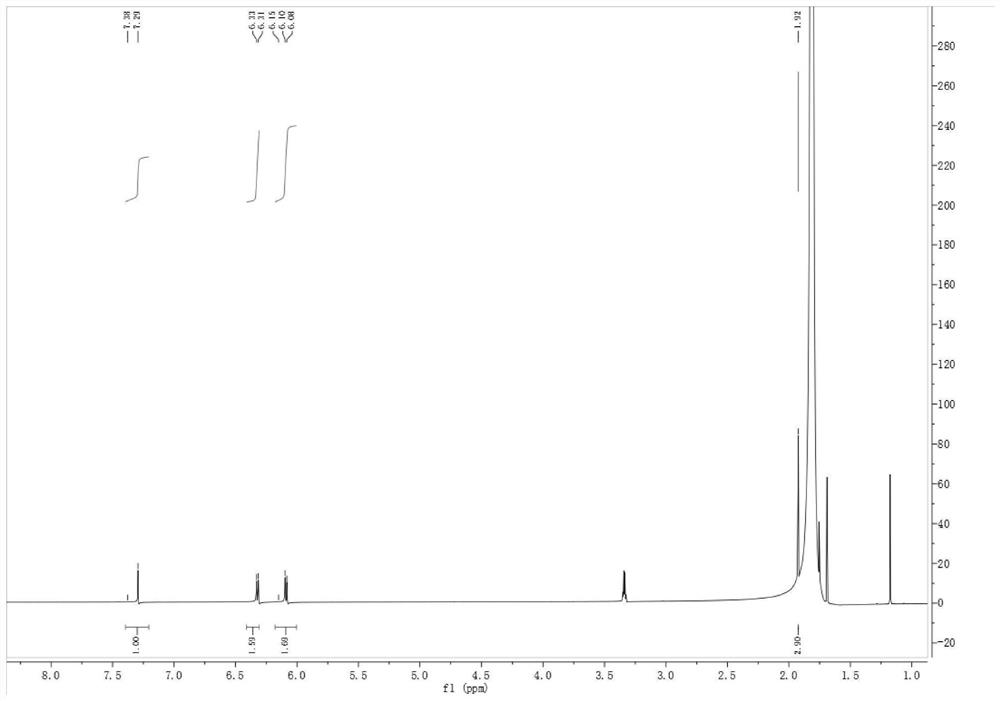
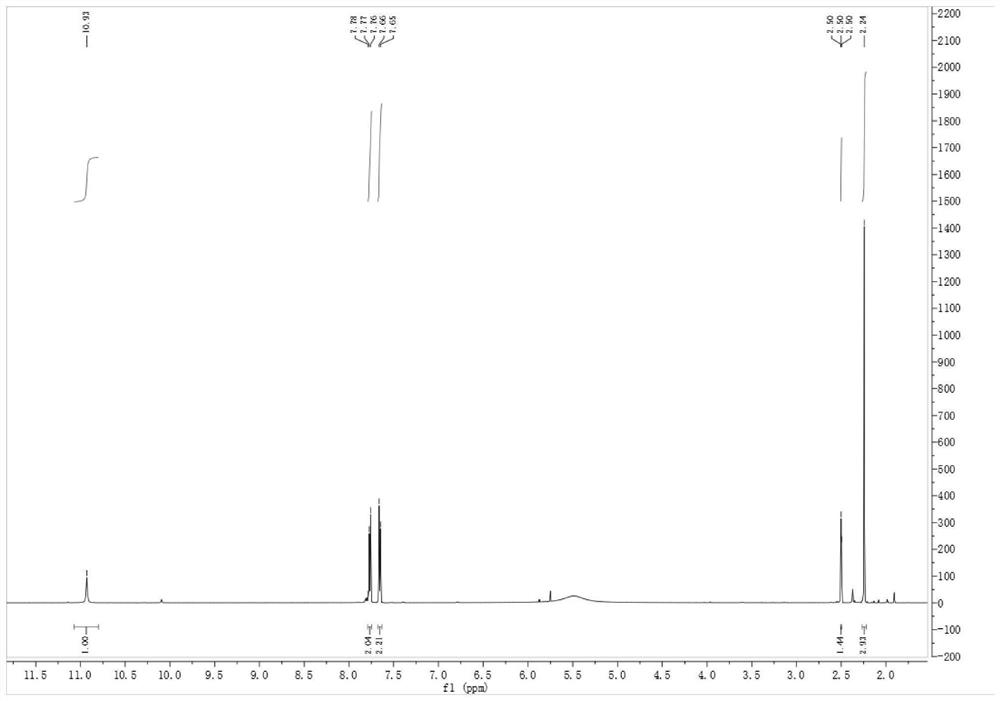
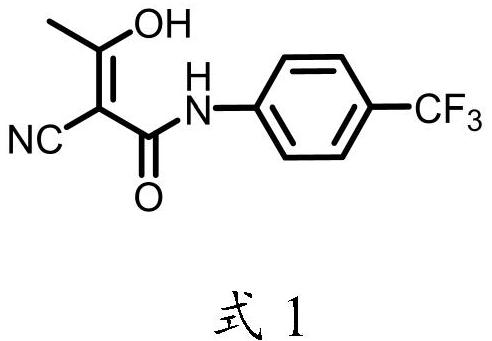
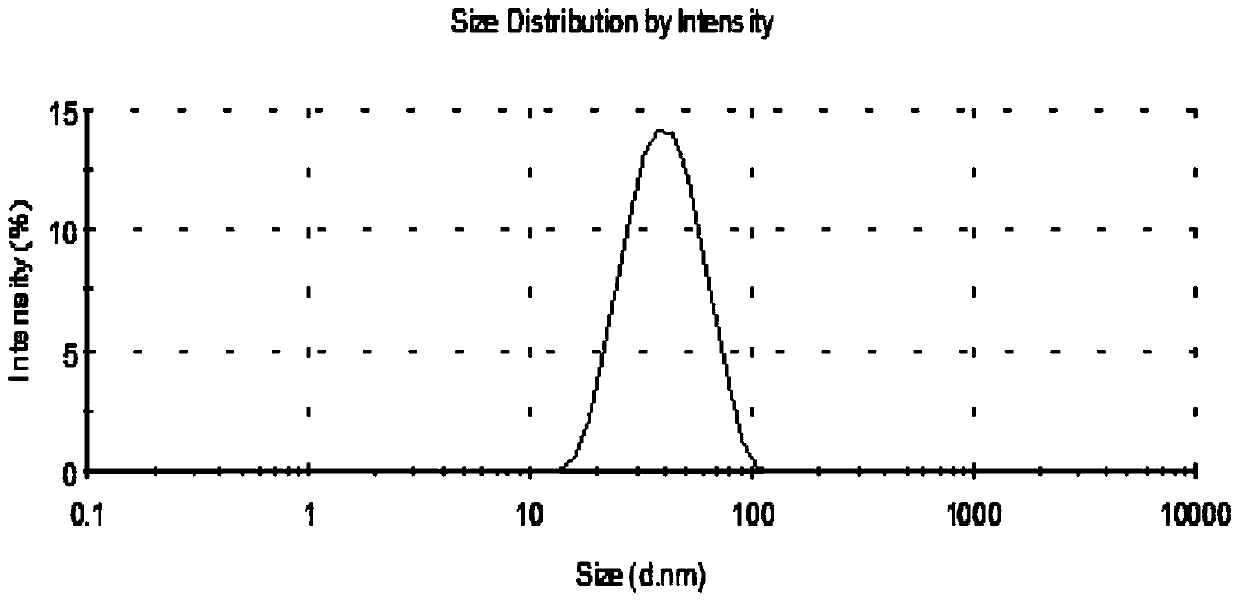
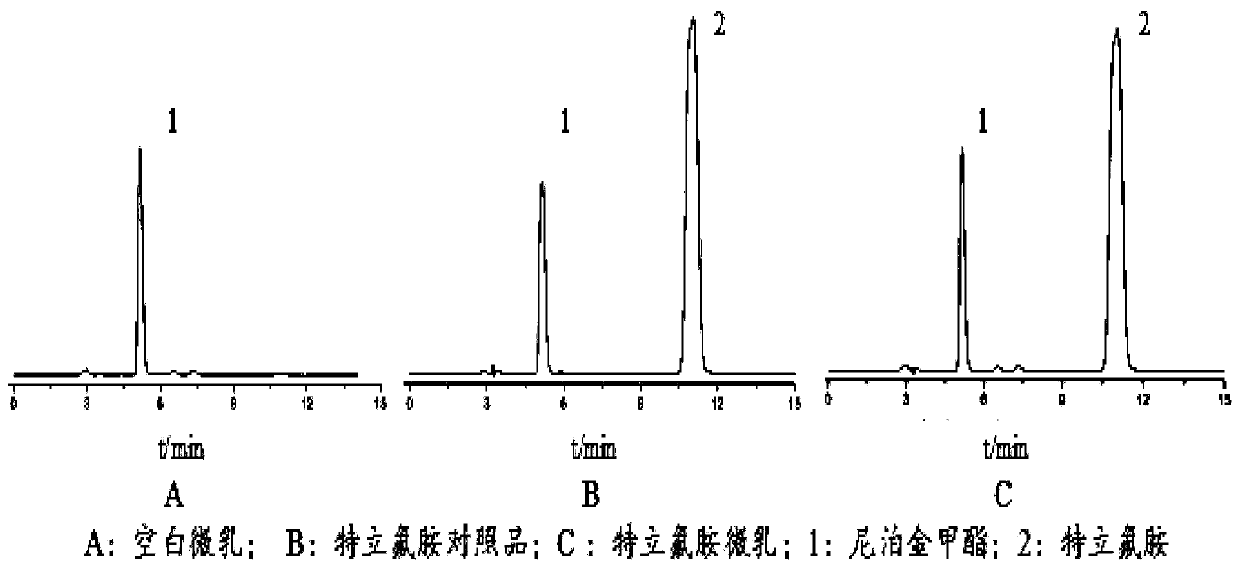
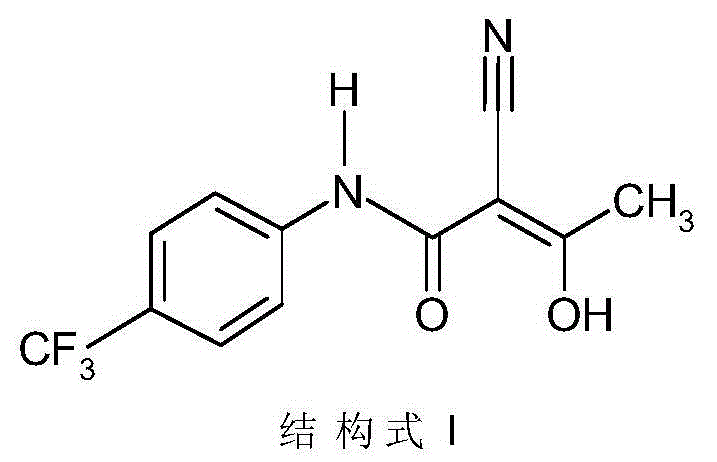
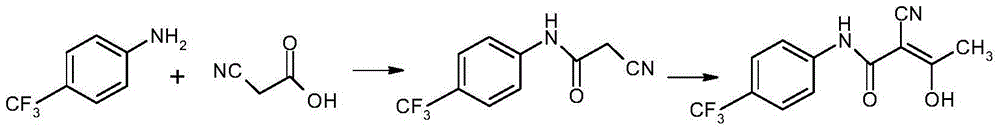
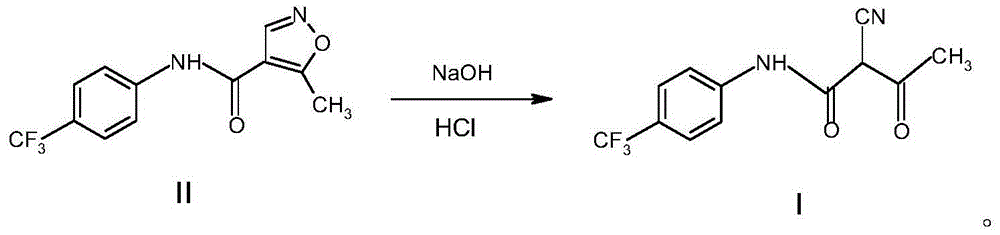
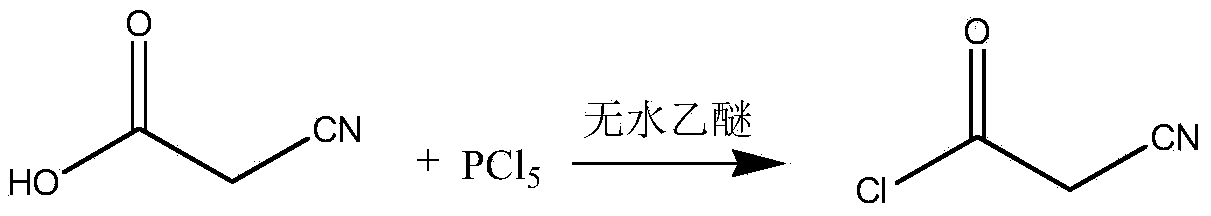Patents
Literature
36 results about "Teriflunomide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
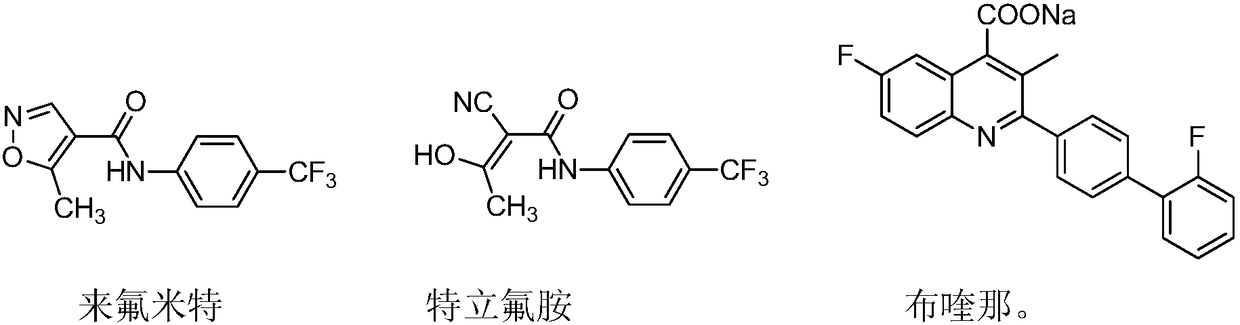
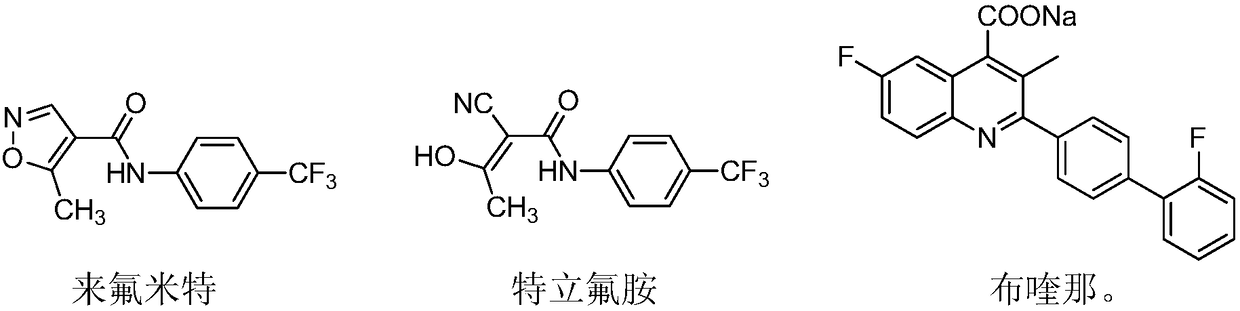
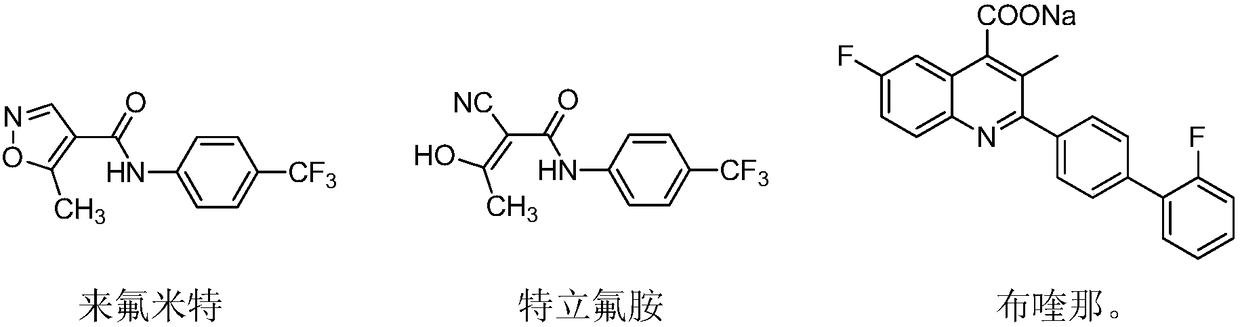
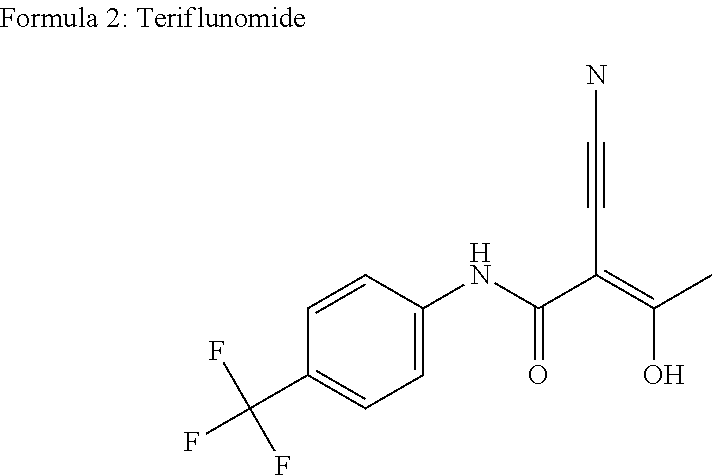
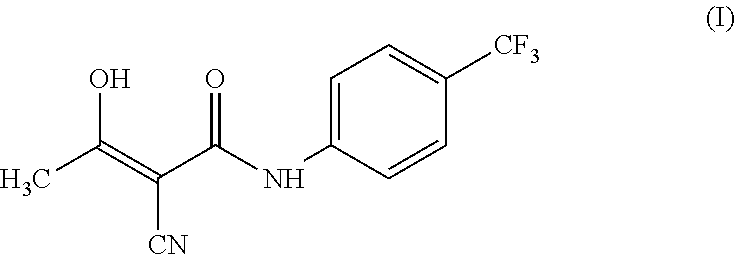
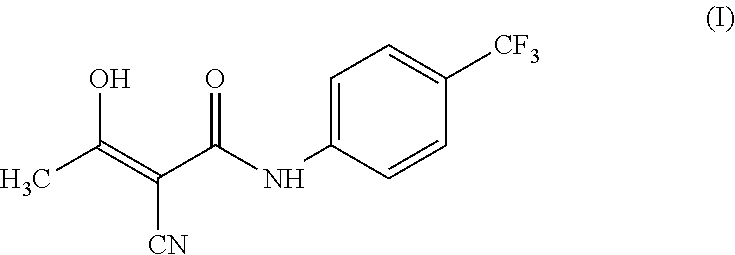
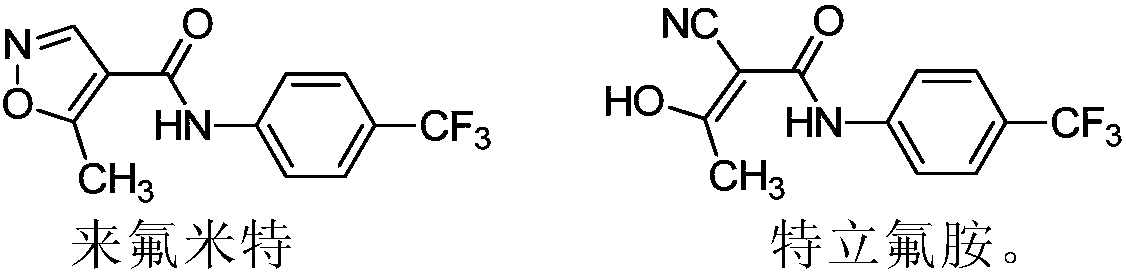
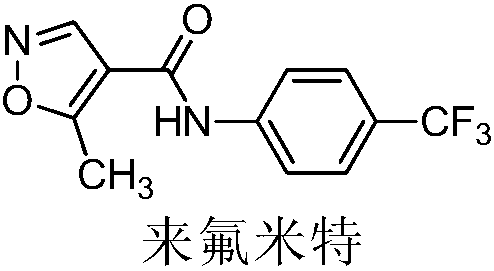
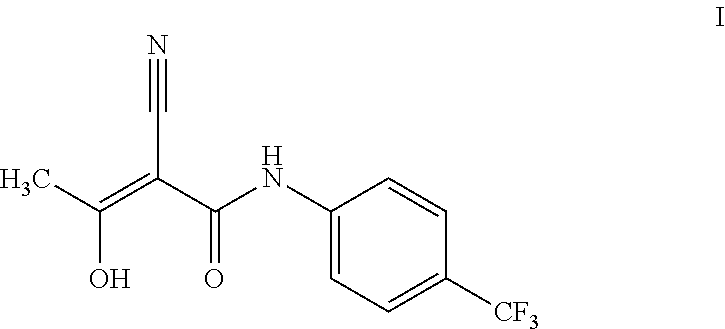
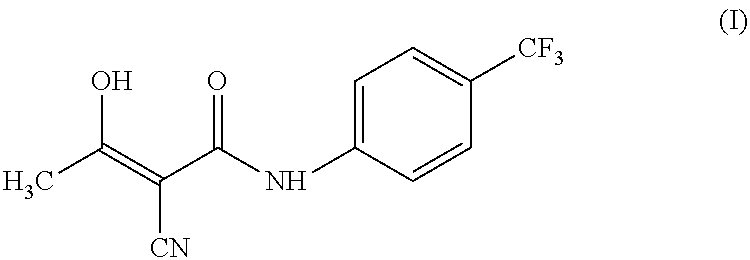
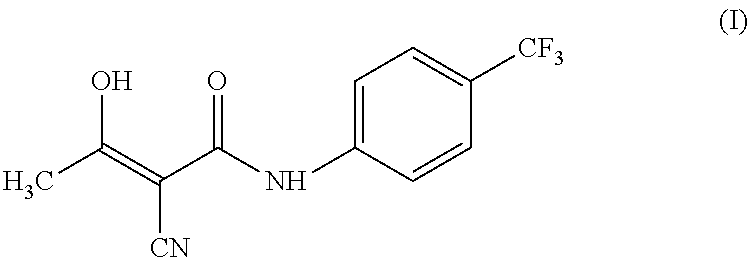
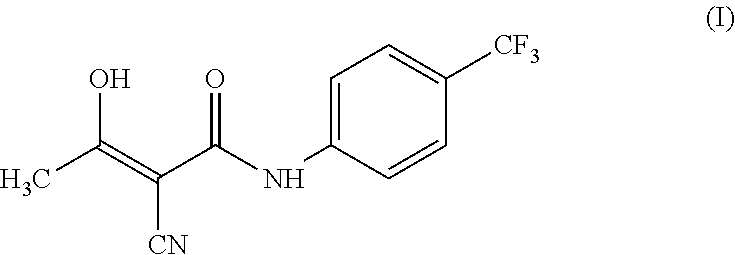
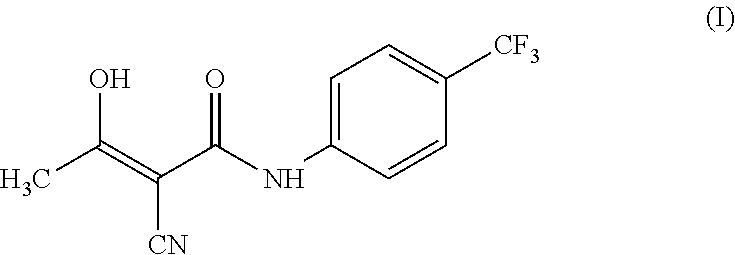
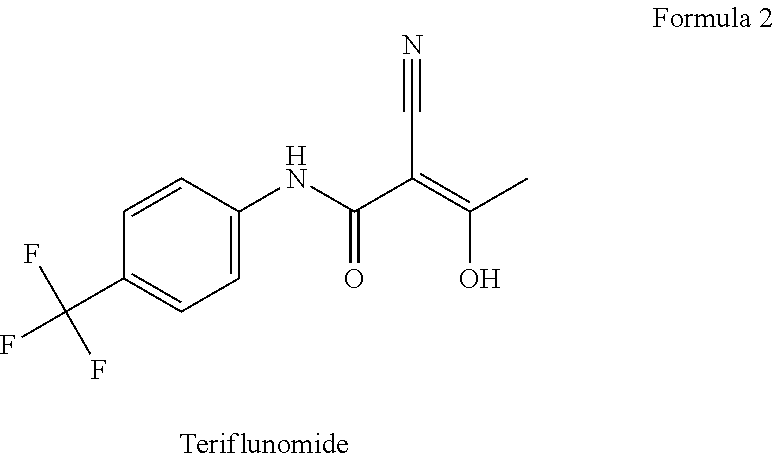
Teriflunomide (trade name Aubagio, marketed by Sanofi) is the active metabolite of leflunomide. Teriflunomide was investigated in the Phase III clinical trial TEMSO as a medication for multiple sclerosis (MS). The study was completed in July 2010. 2-year results were positive. However, the subsequent TENERE head-to-head comparison trial reported that "although permanent discontinuations [of therapy] were substantially less common among MS patients who received teriflunomide compared with interferon beta-1a, relapses were more common with teriflunomide." The drug was approved by the FDA on September 13, 2012 and in the European Union on August 26, 2013.
Novel antiviral medicines and application thereof
PendingCN108721281ABroad-spectrum and excellent antiviral activityLow toxicityAntiviralsNitrile/isonitrile active ingredientsLeflunomideRNA virus
The invention discloses application of leflunomide, teriflunomide, brequinar and derivatives thereof to treatment of virus infection, especially RNA virus infection. RNA viruses include but are not limited to influenza viruses, respiratory syncytial viruses, hand, foot and mouth viruses (EV71), dengue viruses (type-2 dengue viruses), Zika viruses and Japanese encephalitis viruses. The medicines have broad-spectrum and excellent antiviral activity and have relatively low toxicity to normal cells.
Owner:EAST CHINA UNIV OF SCI & TECH
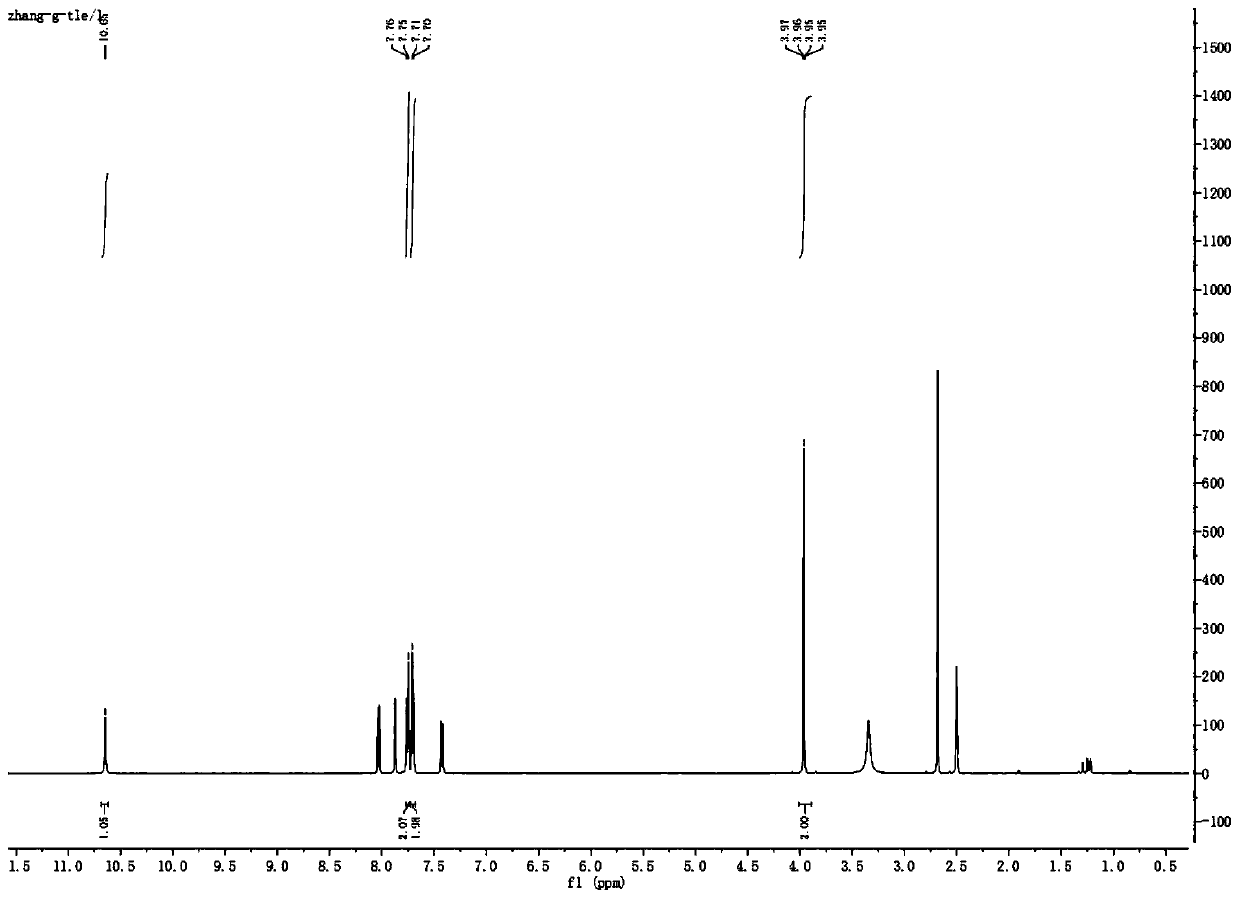
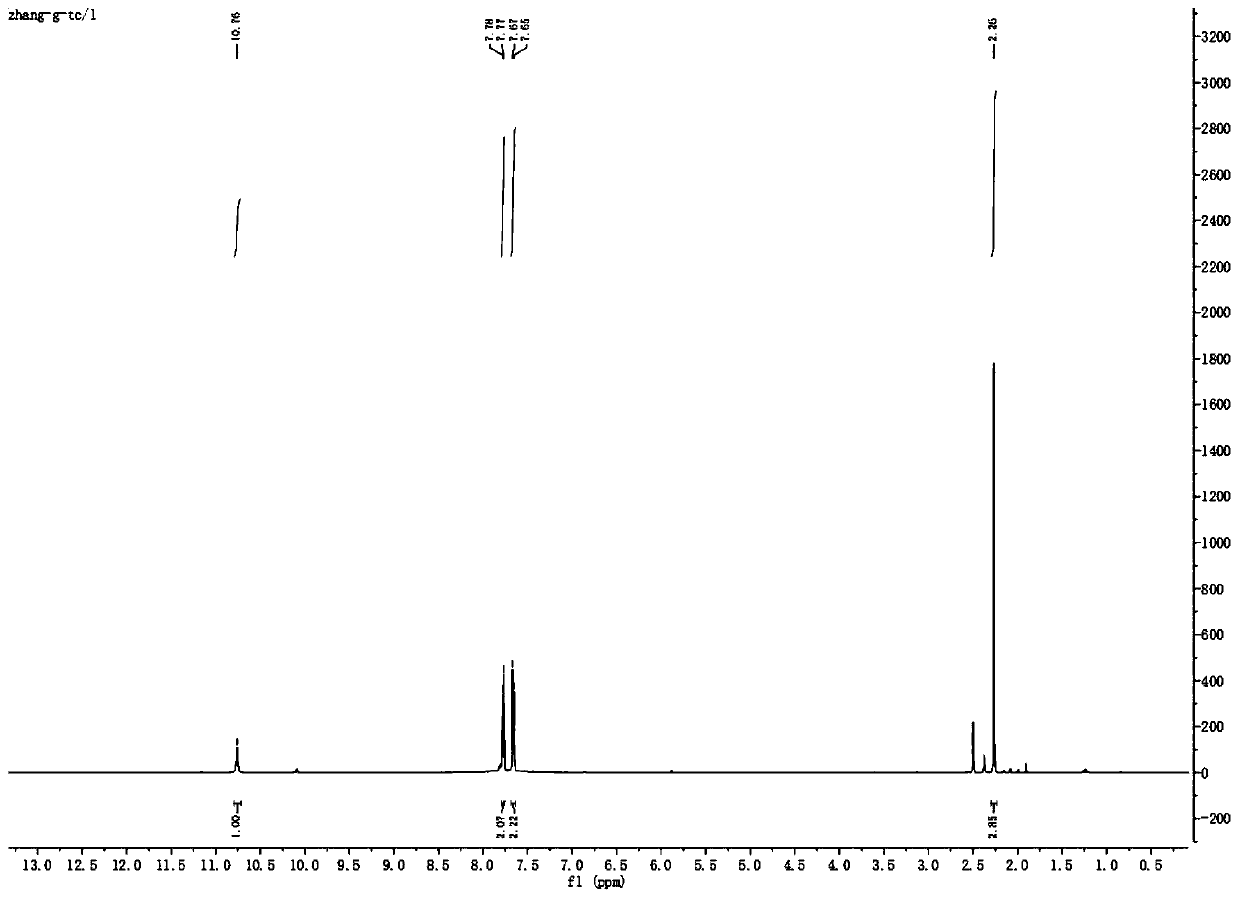
Preparation method of teriflunomide
InactiveCN102786437AImprove water stabilitySimple and fast operationCarboxylic acid nitrile preparationOrganic compound preparationAcetic anhydrideCyanoacetic acid
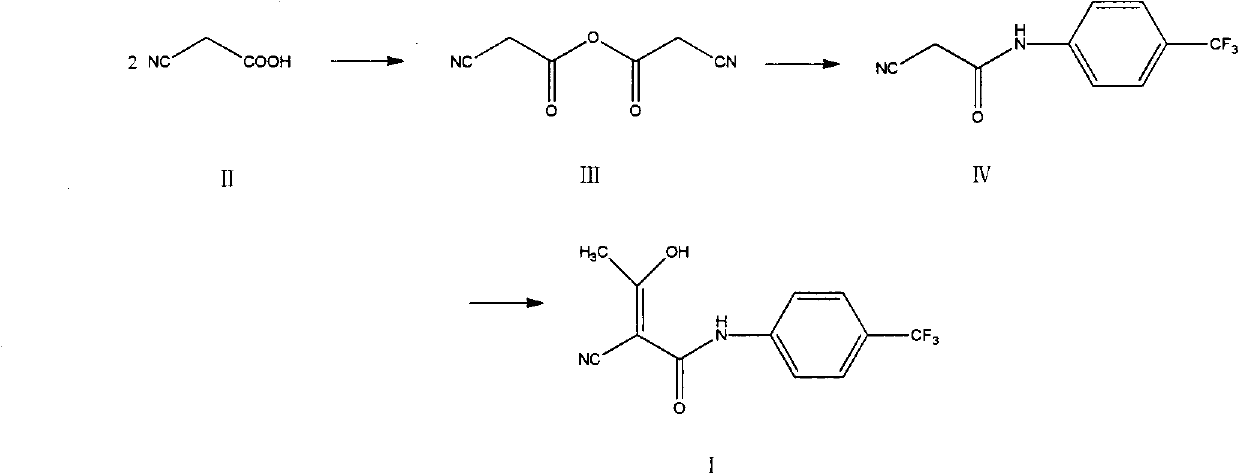
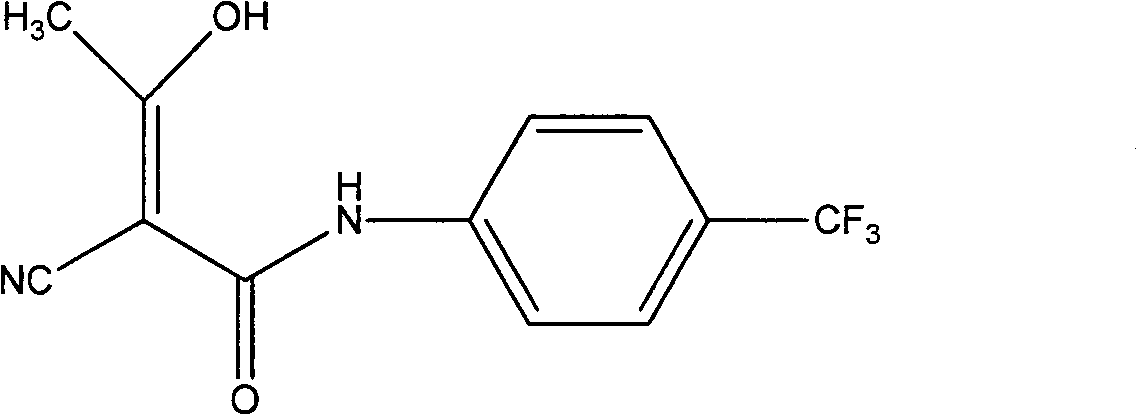
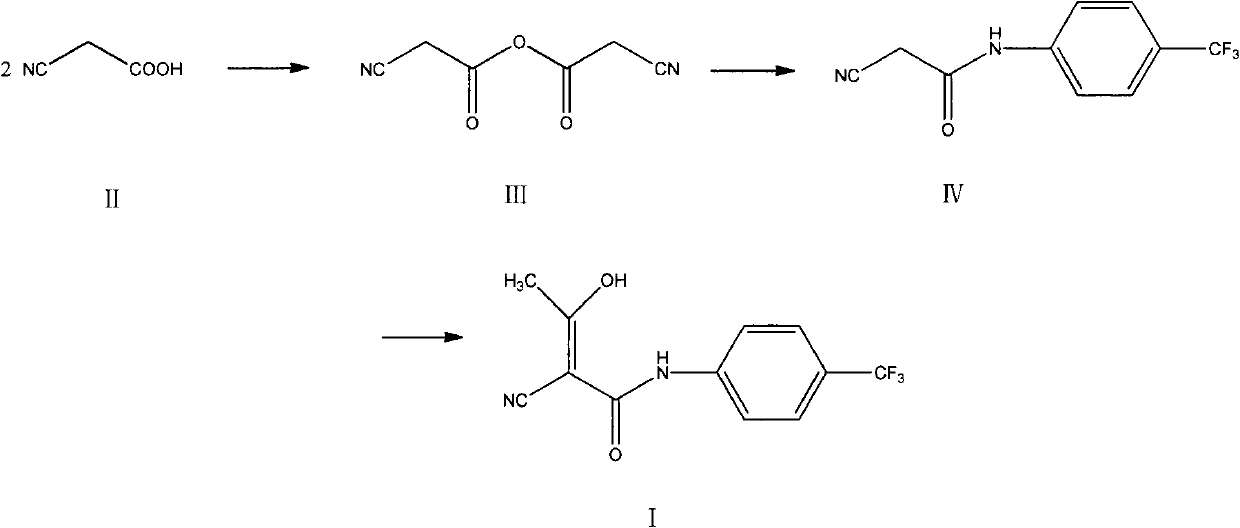
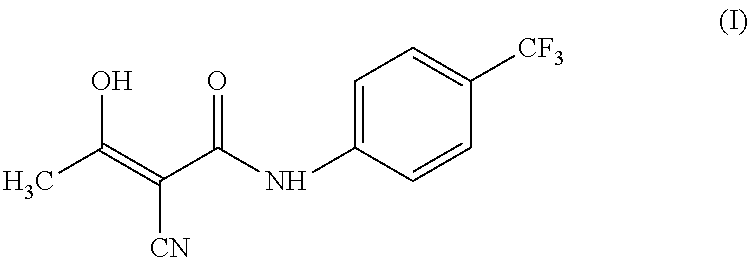
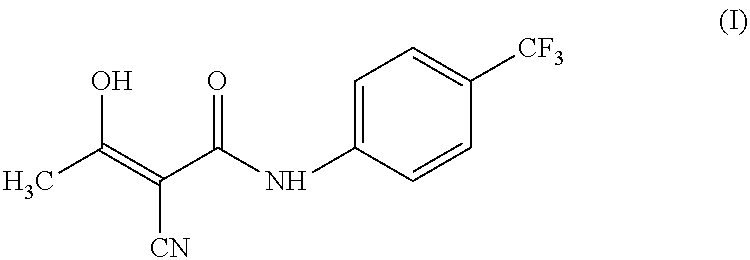
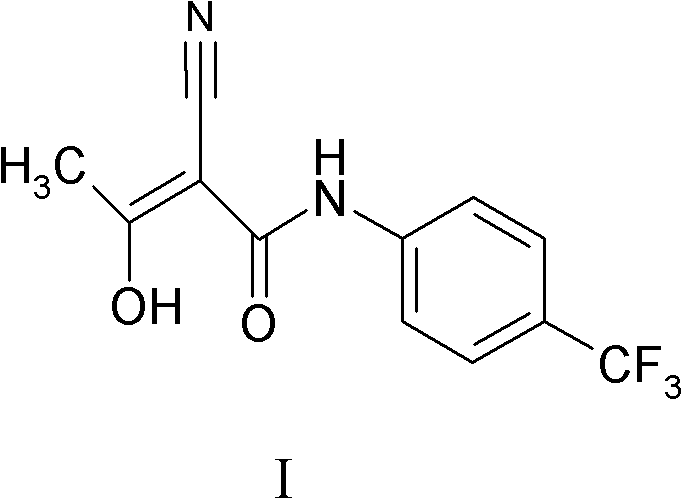
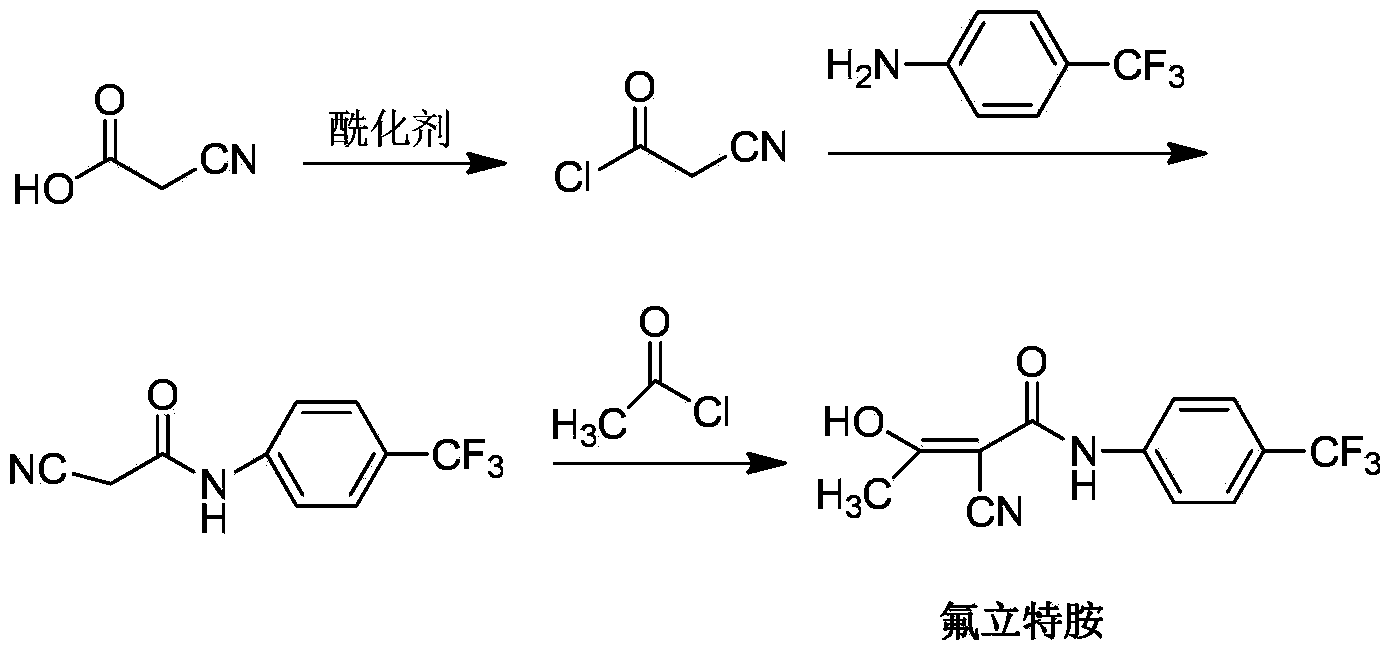
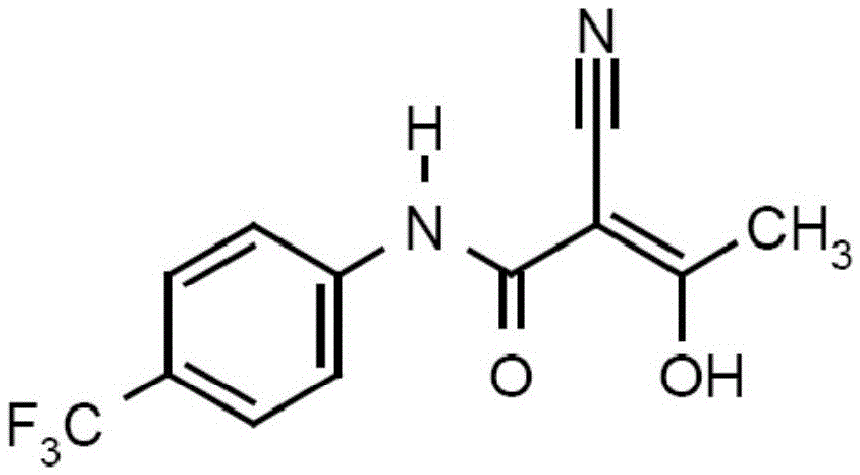
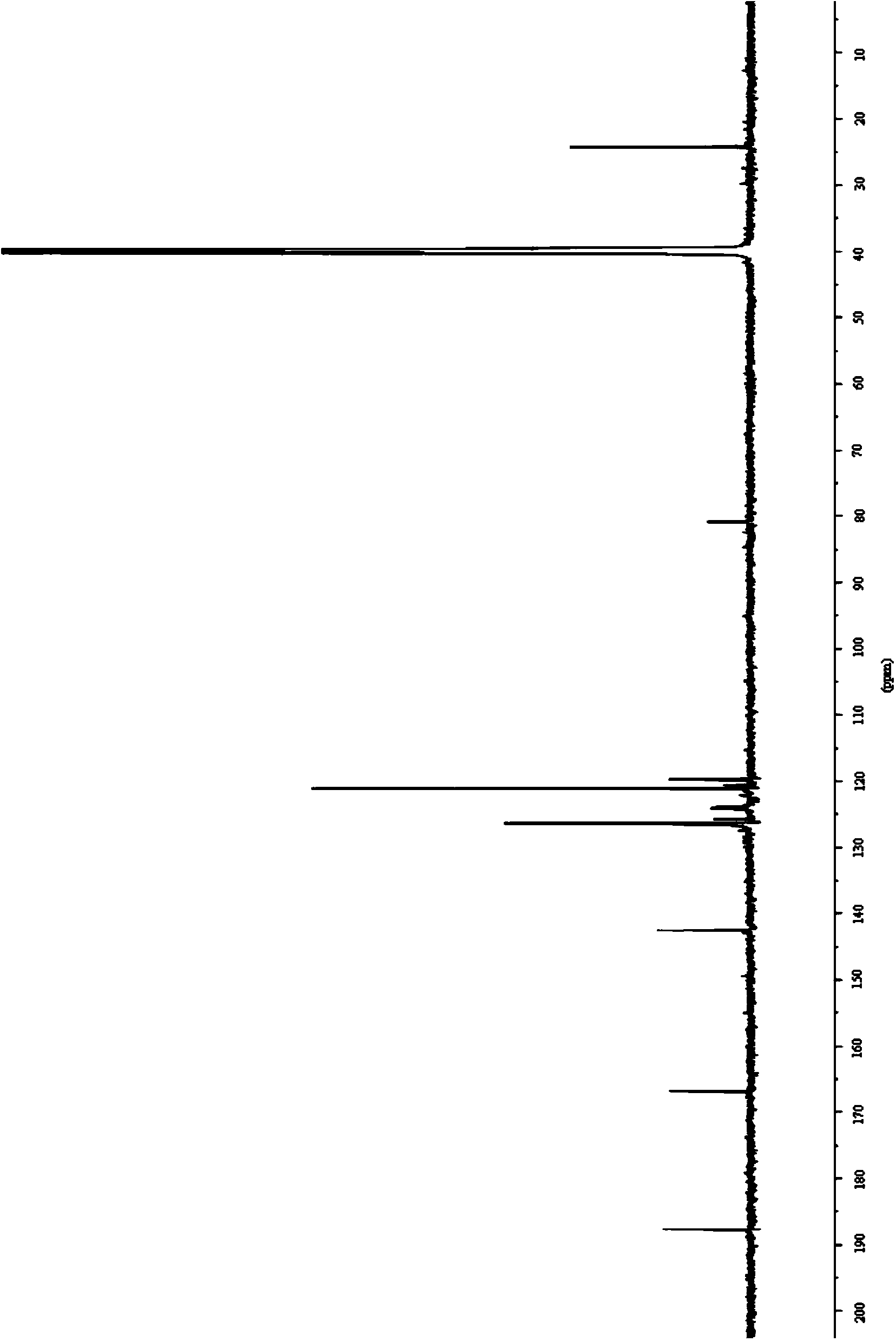
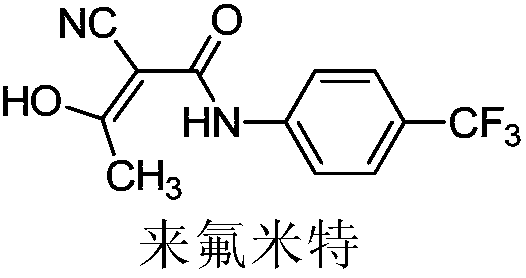
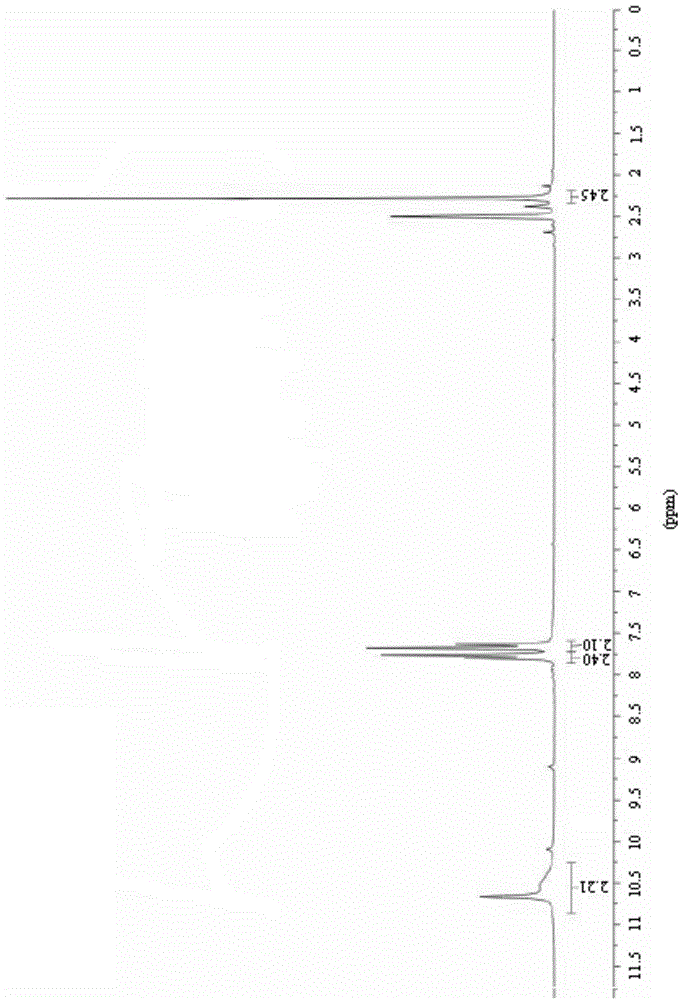
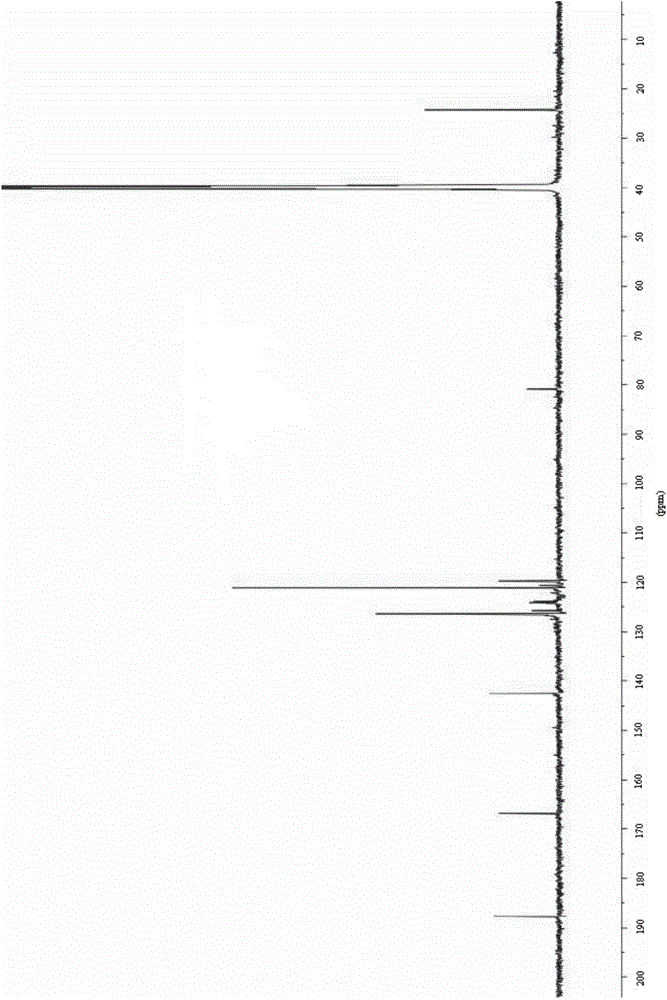
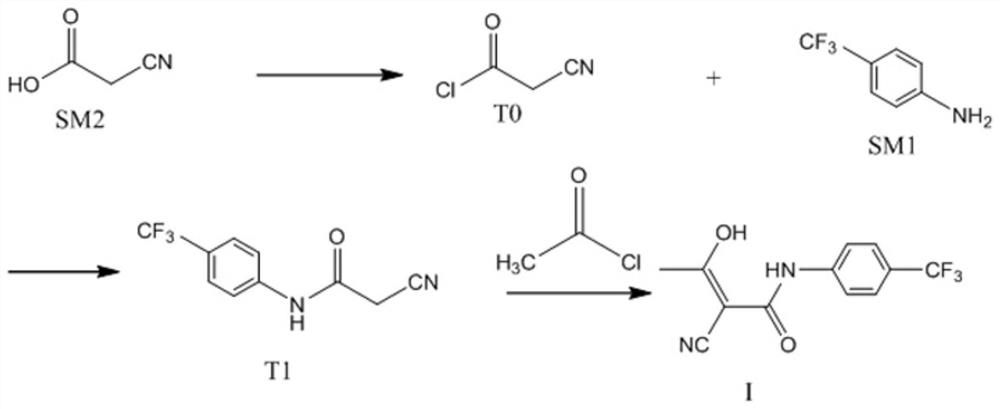
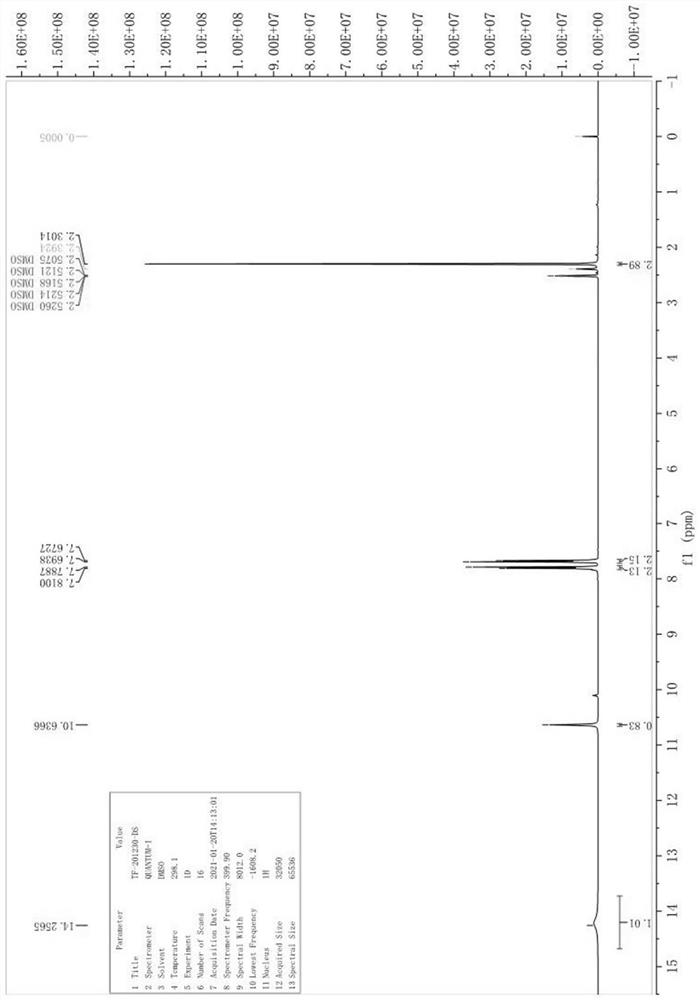
The invention relates to a preparation method of (Z)-2-cyano-3-hydroxy-N-(4-(trifluoromethyl)phenyl)-2-butenamide (I). The preparation method is characterized in that cyanoacetic acid (I) is dehydrated and condensed to obtain cyan acetic anhydride (III), the cyan acetic anhydride (III) reacts with trifluoromethyl phenyl under the action of catalyst to obtain 2-cyano-N-(4-trifluoromethyl-phenyl)-ethanamide (IV), and the 2-cyano-N-(4-trifluoromethyl-phenyl)-ethanamide (IV) reacts with the acetylchloride in the presence of sodium hydride to obtain teriflunomide (I).
Owner:CHINA PHARM UNIV
Process for preparing teriflunomide
InactiveUS20110092727A1Easy to operateEasy to handleCarboxylic acid nitrile preparationOrganic compound preparationPolymer scienceTeriflunomide
Owner:ALEMBIC LTD
Compound teriflunomide transdermal patch for treating rheumatic arthritis, and preparation method thereof
ActiveCN103520137AImprove adhesionImprove flexibilityAntipyreticAnalgesicsTransdermal patchPharmaceutical drug
The invention discloses a compound teriflunomide transdermal patch for treating rheumatic arthritis, and a preparation method thereof, and belongs to the technical field of medicines. The compound teriflunomide transdermal patch for treating rheumatic arthritis is composed of a back lining layer, a medicine storage layer and an anti-adhesive layer, wherein the medicine storage layer is mainly prepared from the following raw material drugs in parts by weigh: 0.55-12 parts of teriflunomide, 0.55-12 parts of non-steroid anti-inflammatory drug, and 100 parts of pressure-sensitive adhesive. The compound teriflunomide transdermal patch can be used for avoiding irritation of an oral drug to gastrointestinal tracts, increasing the percutaneous permeability of the drug by an ion-pair technology, has a durable and stable curative effect, and can be used for treating rheumatic arthritis and pains caused by the same; if administration is interrupted, the patch can be peeled off, and the use is convenient; the patch is good in adhesion and flexibility.
Owner:SHENYANG PHARMA UNIVERSITY
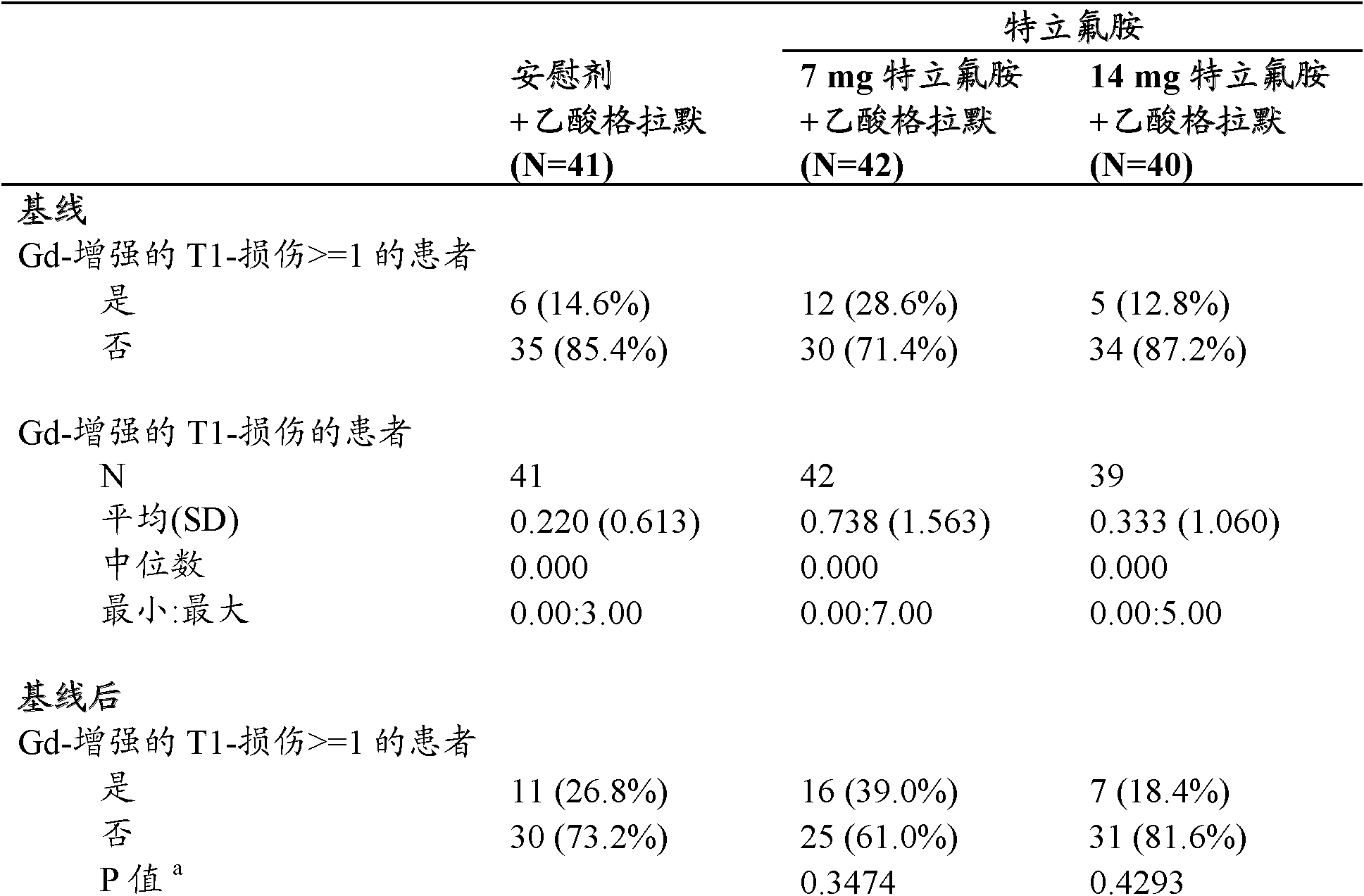
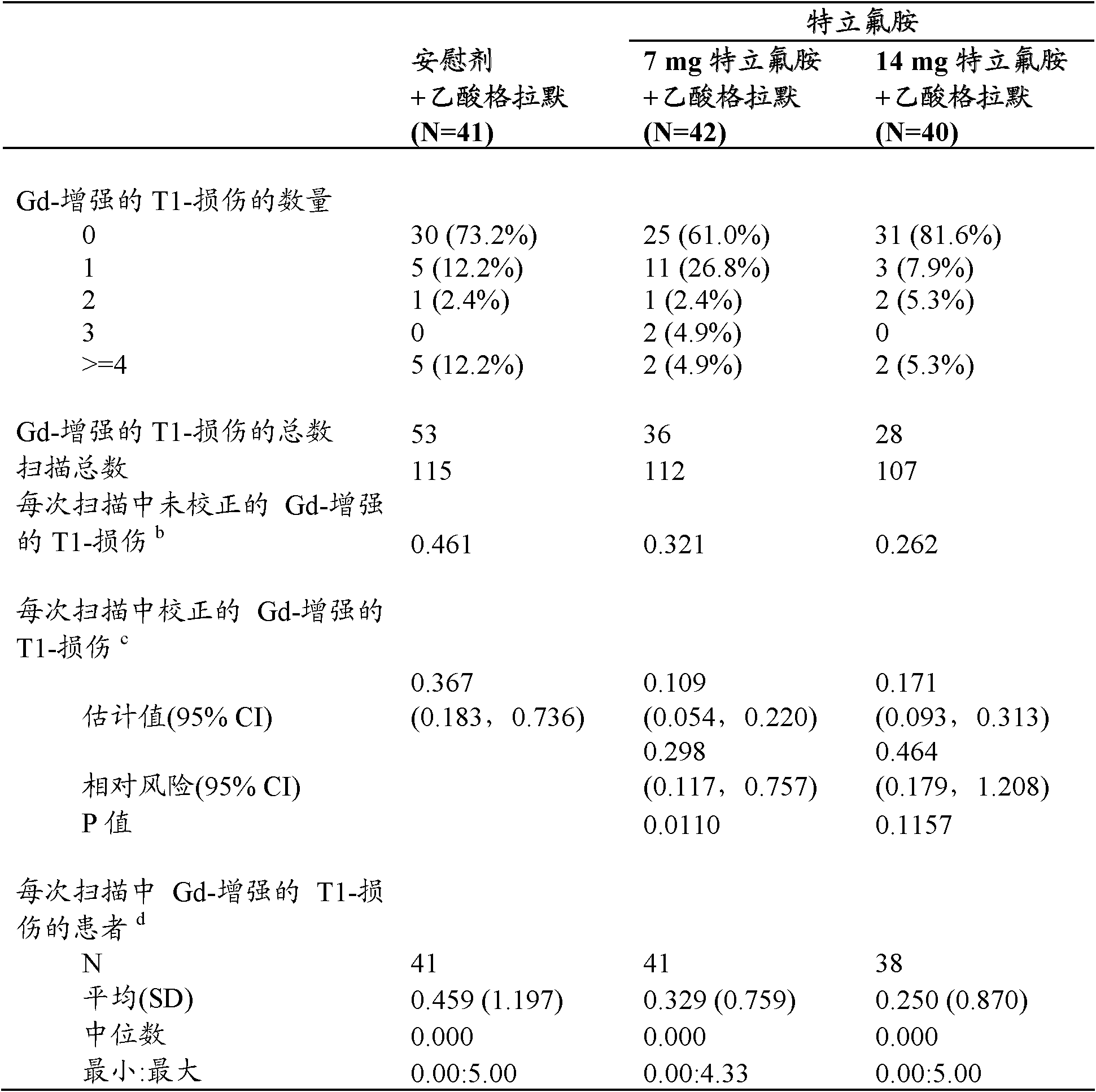
Use of the combination of teriflunomide and glatiramer acetate for treating multiple sclerosis
This invention is related to the use of the combination of teriflunomide or a pharmaceutically acceptable salt thereof and glatiramer acetate for treating multiple sclerosis.
Owner:SANOFI AVENTIS US LLC
Preparation method of teriflunomide
InactiveCN110903214AHigh yieldHigh reactivityCarboxylic acid nitrile preparationOrganic compound preparationCyanoacetic acidPhenyl group
The invention relates to the technical field of medicinal chemistry, in particular to a preparation method of teriflunomide. The preparation method includes: (1) mixing cyanoacetic acid, a condensingagent, an aprotic solvent and an alkaline reagent, and carrying out condensation reaction to obtain an active ester system; (2) mixing the active ester system with 4-trifluoromethylaniline, and carrying out condensation reaction to obtain an intermediate 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide; and (3) mixing the intermediate 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide with acetyl chloride, and carrying out acylation reaction to obtain teriflunomide. According to the preparation method, cyanoacetic acid is reacted with 4-trifluoromethylaniline in the form of active ester, the reaction activity of cyanoacetic acid and 4-trifluoromethylaniline is improved, the reaction conditions are mild, the obtained active ester system can directly react with 4-trifluoromethylaniline without purification and post-treatment, the intermediate purification step is avoided, and the yield of teriflunomide is increased.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
Preparation method of teriflunomide
InactiveCN103709068ARaw materials are easy to getMild reaction conditionsCarboxylic acid nitrile preparationOrganic compound preparationAcetyl chloridePara-trifluoromethylaniline
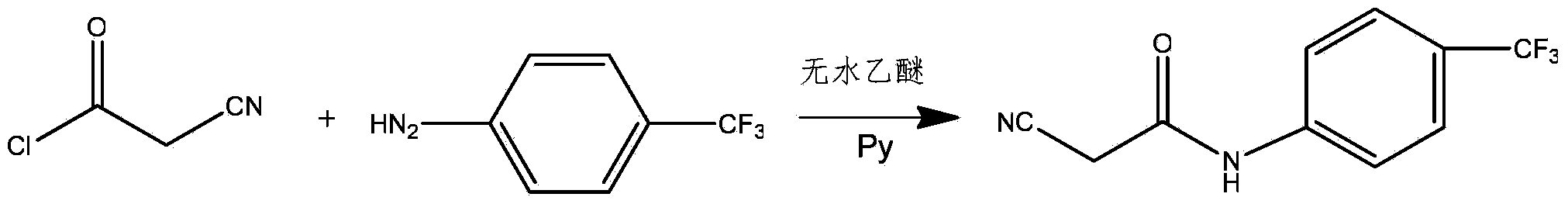
The invention relates to a preparation method of teriflunomide. The preparation method comprises the following steps: mixing cyano acetyl chloride, p-trifluoromethylaniline and an acid-binding agent according to the molar ratio of 1:(1-3):(8-11), and reacting to obtain 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide; mixing 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide, acetyl chloride and an acid-binding agent according to the molar ratio of 1:(1-5):(1-5) of reaction materials, and reacting to obtain teriflunomide. The process has the advantages that the raw materials are easily available, the reaction condition is mild and the yield is high, and the preparation method is suitable for industrial production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
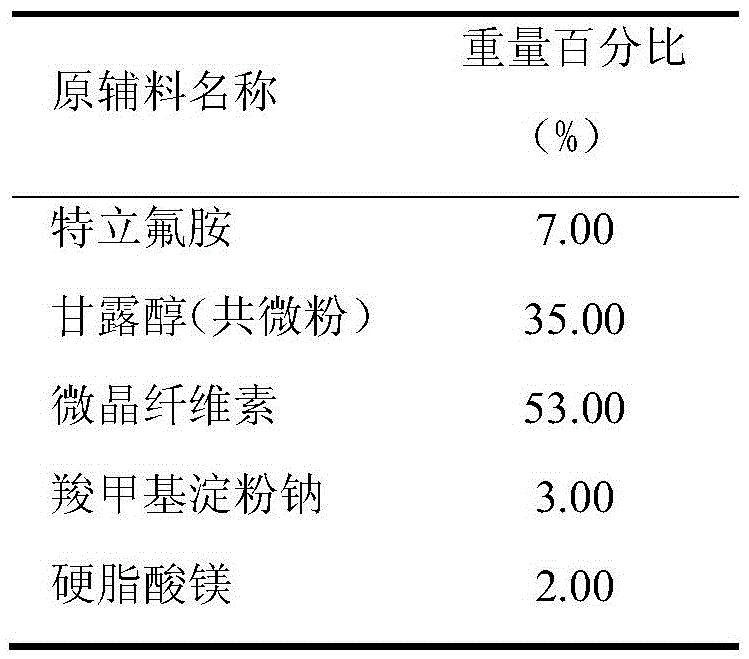
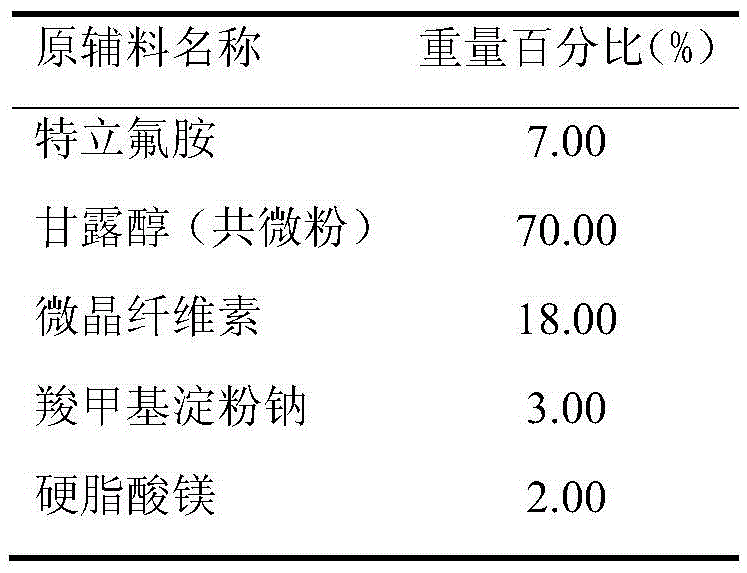
Teriflunomide dispersible tablet and preparation method thereof
InactiveCN106880608AThere are few varieties to chooseImprove securityNervous disorderPill deliveryMedicineDissolution
The invention provides a teriflunomide dispersible tablet and a preparation method thereof. The dispersible tablet consists of teriflunomide, which serves as an active ingredient, and a filler, a disintegrating agent and a lubricating agent which are used as pharmaceutical adjuvants. The teriflunomide dispersible tablet prepared by the invention can be completely disintegrated rapidly, so that a time to peak is shortened; a dissolution rate can reach 90% or above; the dispersible tablet is convenient to take, rapid in efficacy release and high in bioavailability; and the preparation method is simple in process, easy for control, convenient to operate and suitable for industrial production.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
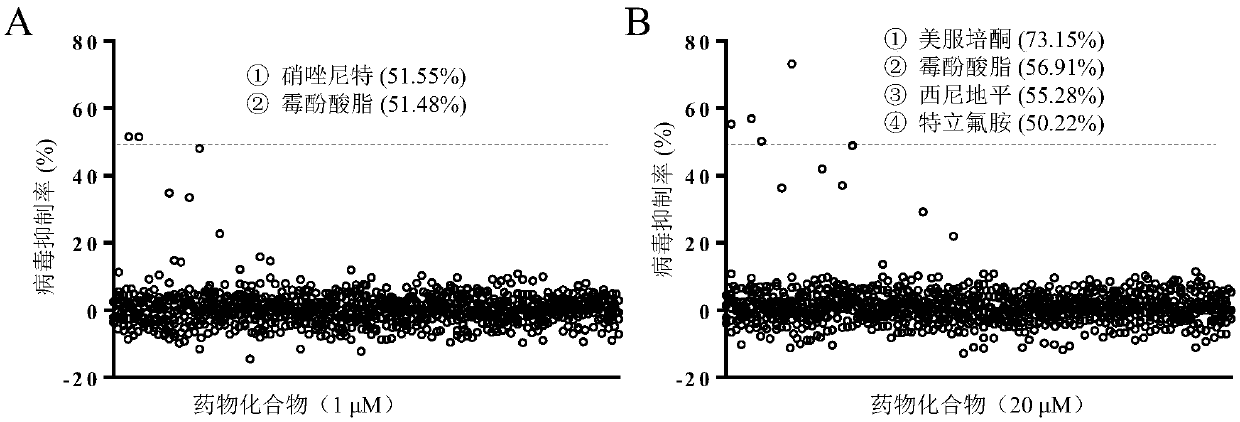
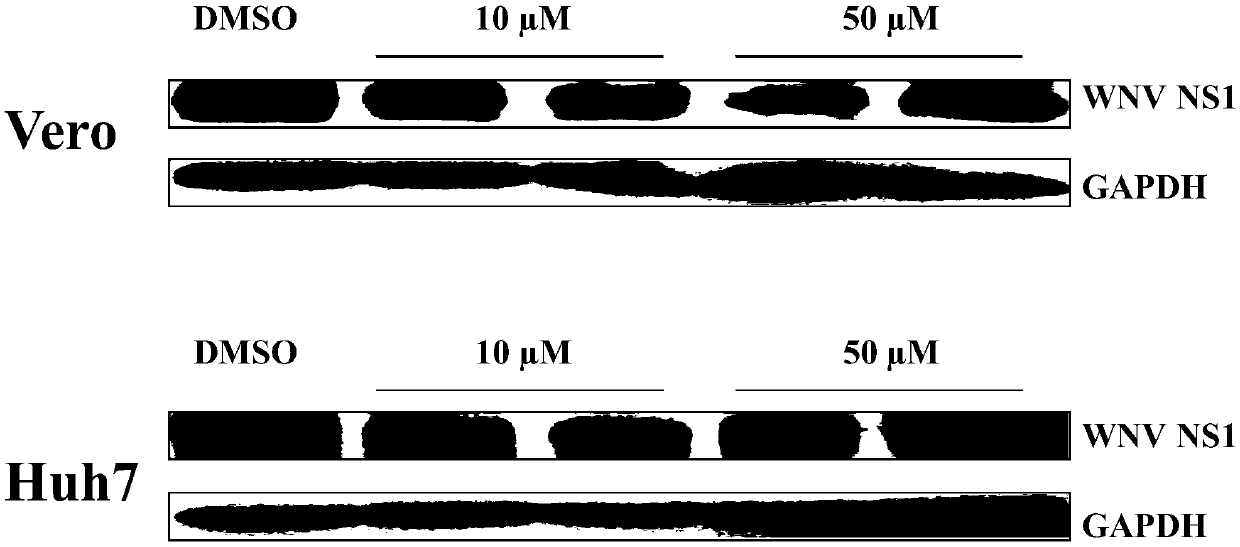
Application of teriflunomide to preparation of anti-west Nile virus medicine
InactiveCN108670965AImprove survival rateAvoid infectionAntiviralsNitrile/isonitrile active ingredientsWest Nile Virus InfectionActive component
The invention discloses application of teriflunomide to preparation of an anti-west Nile virus medicine. The anti-west Nile virus medicine takes the teriflunomide as a single active component or is amedicine composition containing the teriflunomide; and the anti-west Nile virus medicine is a medicine for preventing or treating west Nile virus infection. A susceptible cell line containing the westNile virus comprises a human liver cancer cell line Huh7, a monkey kidney cell Vero and a west Nile virus infected mouse model, and is used for detecting the anti-west Nile virus activity of the teriflunomide. The experimental result shows that the teriflunomide can effectively inhibit infection on the above susceptible cell and the animal model by the west Nile virus as well as proves that the teriflunomide can effectively inhibit west Nile virus from infecting a targeted cell, can increase the survival rate of the west Nile virus infected mice, has low toxicity, can serve as a potential anti-west Nile virus medicine and has further development prospect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Application of sodium teriflunomide to preparation of medicine for treating autoimmune diseases
InactiveCN105395539AFast absorptionImprove bioavailabilityNervous disorderAntipyreticActive componentAutoimmune disease
The invention discloses an application of sodium teriflunomide, which is a sodium salt with low hepatotoxicity of a leflunomide active metabolite, to preparation of a medicine for treating autoimmune diseases. Compared with leflunomide or teriflunomide, sodium teriflunomide has lower hepatotoxicity and a larger therapeutic window. Sodium teriflunomide or a medical combination taking sodium teriflunomide as an active component is used as an immunosuppressor, and is used for treating autoimmune diseases.
Owner:CINKATE PHARMA INTERMEDIATES
Method for the preparation of teriflunomide
InactiveUS20170073304A1Feasible at commercial scaleCarboxylic acid nitrile preparationOrganic compound preparationAlcoholAqueous sodium hydroxide
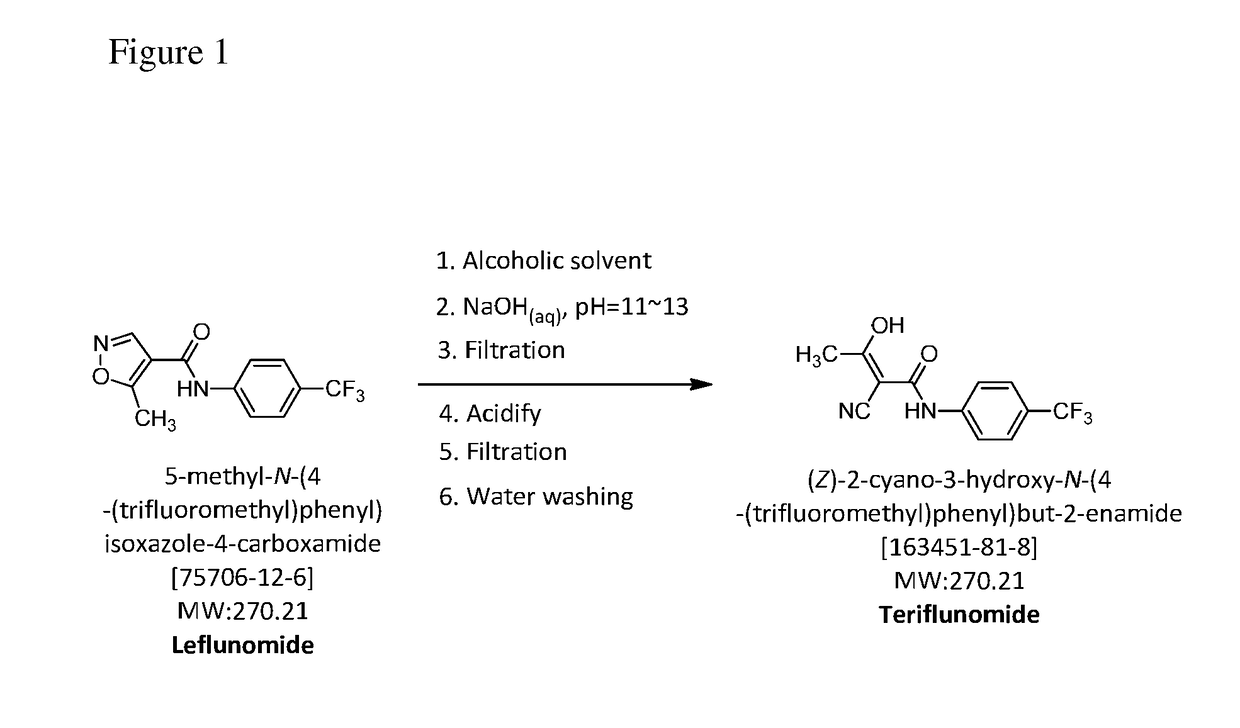
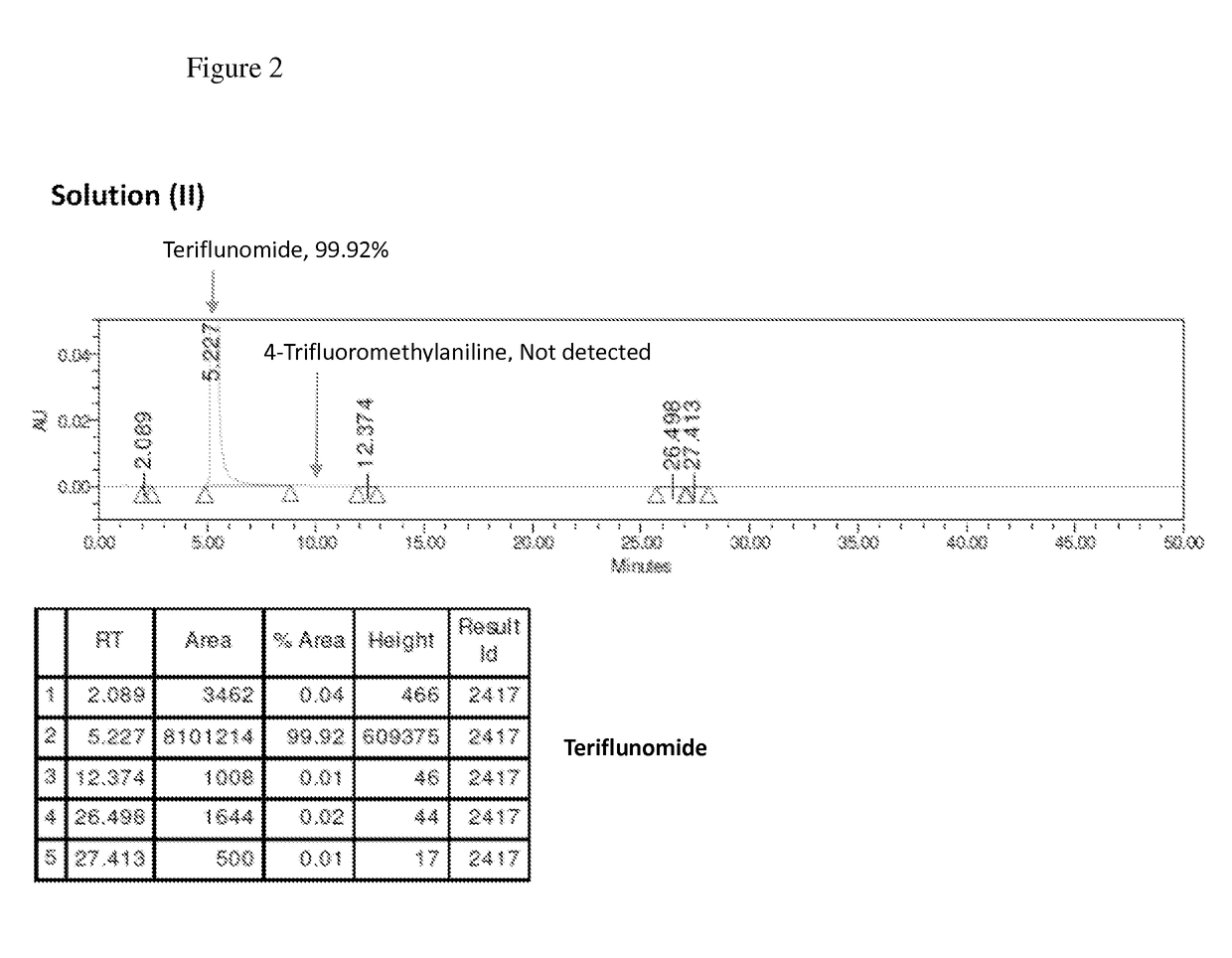
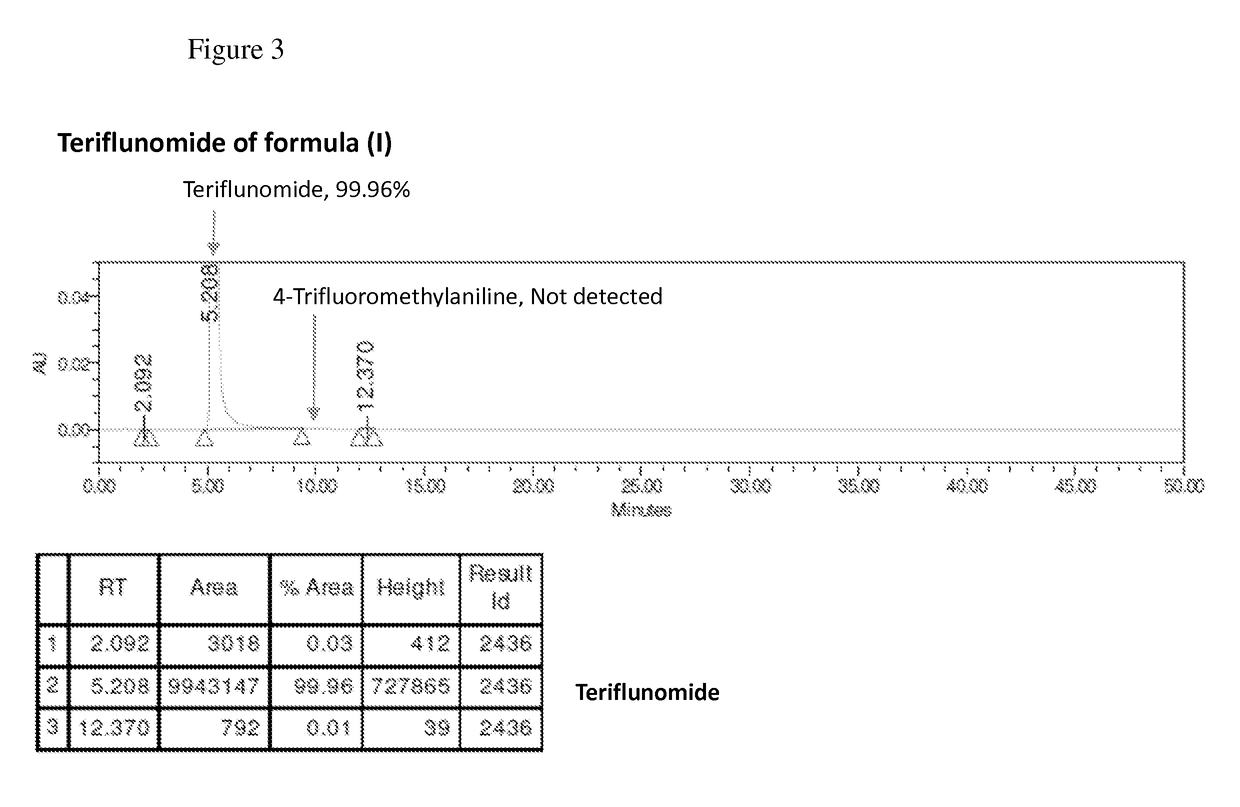
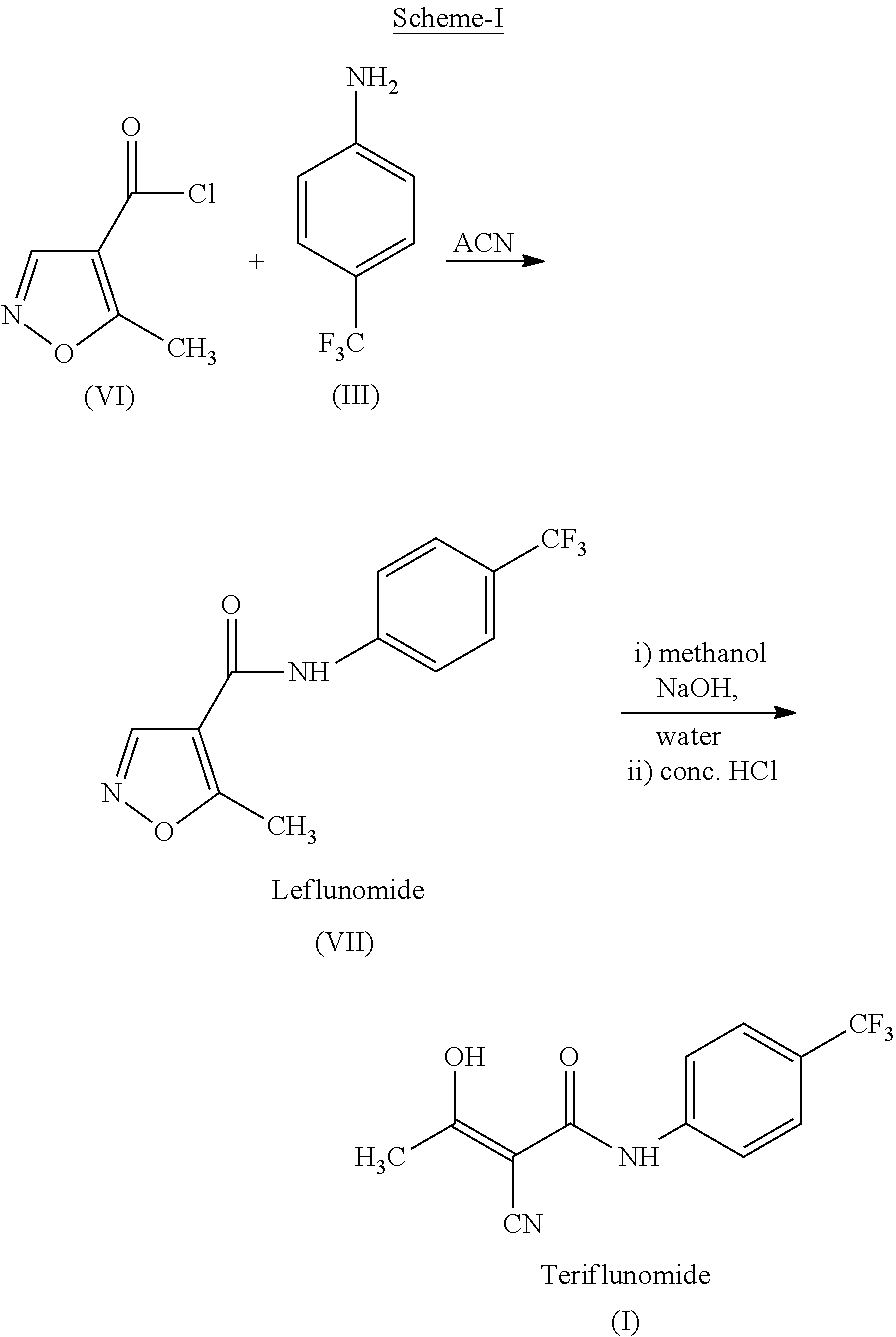
The present invention relates to a method for preparation of Teriflunomide, comprising steps of: (a) adding Leflunomide to an alcoholic solvent to give solution (I); (b) adding an aqueous sodium hydroxide solution slowly into the solution (I) to give solution (II); (c) acidifying the solution (II) with inorganic acid for precipitation to give solution (III); and (d) filtering the solution (III) to give Teriflunomide.
Owner:FORMOSA LAB
Combination therapy for treatment of multiple sclerosis
InactiveUS20150164849A1Good effectIncrease in severe adverse eventBiocideNervous disorderSide effectTolerability
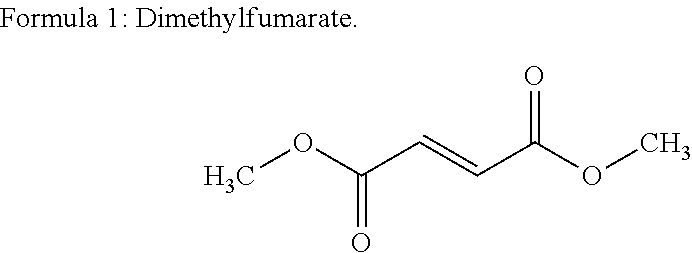
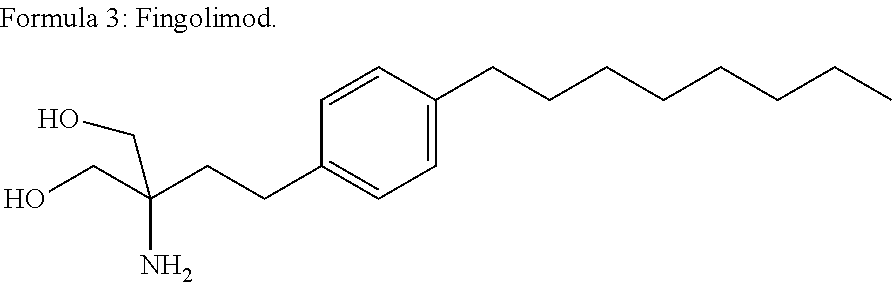
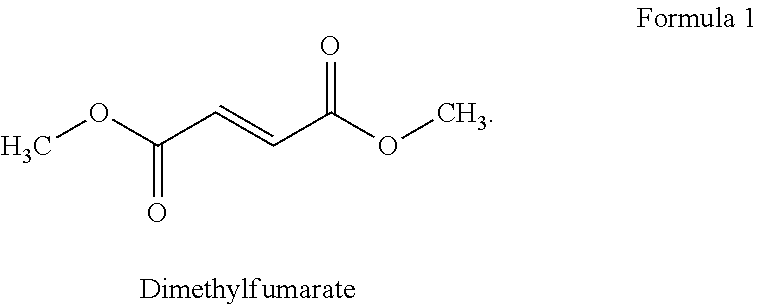
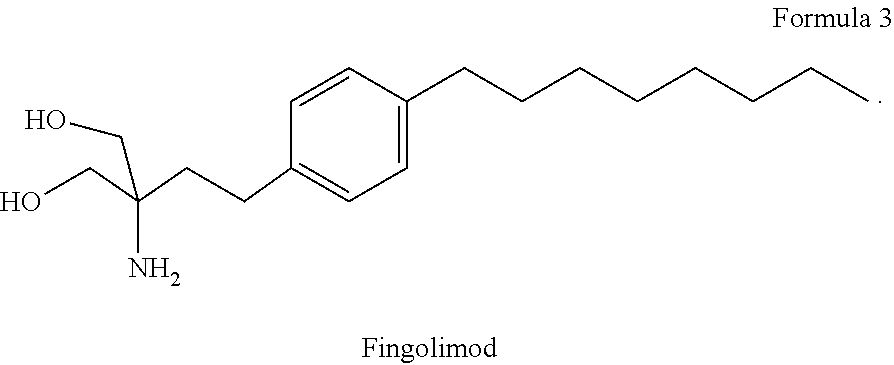
The present invention relates to a method of treating MS in a human patient in need of such treatment and comprises administering to said patient a combination therapy in a single oral dosage form (e.g. a tablet or capsule) of dim ethyifurrta rate and one agent selected from teriflunomide (or its prodrug leflunomide), fingolimod and laquinimod. This combination is more effective than the single agents alone and / or has reduced side effects and better tolerability than the single agents alone and / or can be given in a reduced frequency. Moreover, the present invention is directed to a pharmaceutical composition suitable for the oral treatment of multiple sclerosis consisting of dimethylfumarate and one agent selected from teriflunomide, fingolimod and laquinimod as active ingredients and one or more pharmaceutically acceptable excipients.
Owner:BIOGEN SWISS MFG GMBH
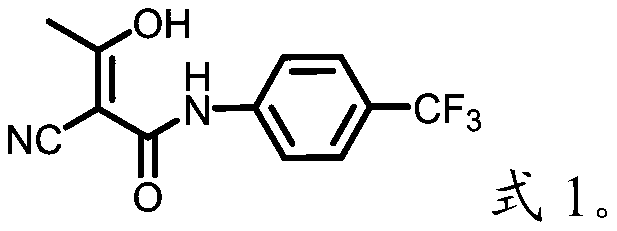
Preparation method of teriflunomide and intermediate thereof
ActiveCN103848756AHigh yieldReduce manufacturing costPreparation from carboxylic acid nitrogen analoguesCarboxylic acid nitrile preparationElectrophilic additionIsomerization
The invention belongs to the technical field of medicinal chemistry and discloses a preparation method of teriflunomide and an intermediate thereof. The preparation method of the teriflunomide provided by the invention comprises the following steps: getting a compound with a structure as shown in formula I and an azide in an organic solvent to generate nucleophilic substitution reaction to generate a compound with the structure as shown in formula II; getting and mixing the compound with the structure as shown in the formula II with the organic solvent, heating to obtain the compound with the structure as shown in formula III; under inert-gas protection and alkaline conditions, getting the compound with the structure as shown in the formula III and the compound with the structure as shown in the formula IV to generate electrophilic addition and isomerization reaction in the organic solvent to obtain the teriflunomide. According to the preparation method of the teriflunomide provided by the invention, the reaction intermediate is not needed to be purified, operation is simple, yield of the teriflunomide is improved, production cost of the teriflunomide is lowered, and therefore, the preparation method is more beneficial to industrial production of the teriflunomide.
Owner:HYBIO PHARMA WUHAN CO LTD
Process for preparation of teriflunomide
InactiveUS20110105795A1Easy to operateOrganic compound preparationPreparation by cyanide reactionPolymer scienceTeriflunomide
Owner:ALEMBIC LTD
Application of leflunomide and teriflunomide to leukemia treatment
InactiveCN108721280AInhibitory activityAntineoplastic agentsNitrile/isonitrile active ingredientsChronic lymphocytic leukemiaChronic granulocytic leukemia
The invention discloses application of leflunomide and teriflunomide or derivatives or pharmaceutically acceptable salt thereof to preparation of medicines for treating leukemia. The leflunomide and the teriflunomid have remarkable inhibition effect on the leukemia comprising acute lymphoblastic leukemia (ALL), acute myelocytic leukemia (AML), chronic granulocytic leukemia, chronic lymphocytic leukemia and the like; and new material basis is laid for the development of the medicines for treating leukemia.
Owner:EAST CHINA UNIV OF SCI & TECH
A kind of preparation method of compound micropill capsule and the prepared compound micropill capsule
InactiveCN104434904BSignificant effectEasy to takeNervous disorderMicrocapsulesSide effectDrug efficiency
The invention belongs to the technical field of medicine preparation, and discloses a preparation method for a compound pellet capsule and the prepared compound pellet capsule. The capsule structure comprises a medicine-carrying pill, a delayed-release layer and a rapid-release layer; the medicine-carrying pill is prepared by employing an extrusion-spheronization process; and the delayed-release layer and the rapid-release layer are prepared by employing a fluidized bed coating process. The prepared capsule is substantial in curative effect, safe, stable, convenient to take, small in side effect and low in production medical cost. The medicine effective compositions dimethyl fumarate and teriflunomide are different in drug action mechanism, also medicine administration is simple and convenient, and the curative effect of the two medicine effective compositions generates synergic effect, the indication scope is larger than that of a single medicine, multiple sclerosis is treated through multiple targets, and the curative effect is better than that of a single medicine.
Owner:HYBIO PHARMA
A novel process for the preparation of teriflunomide
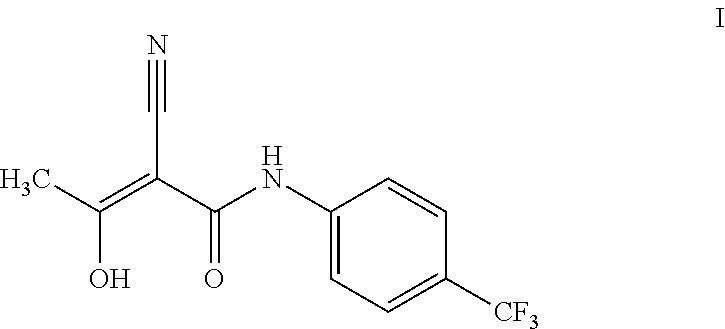
ActiveUS20180170859A1High purityHigh yieldMaterial analysis using wave/particle radiationOrganic compound preparationCarbonyl chlorideCarboxylic acid
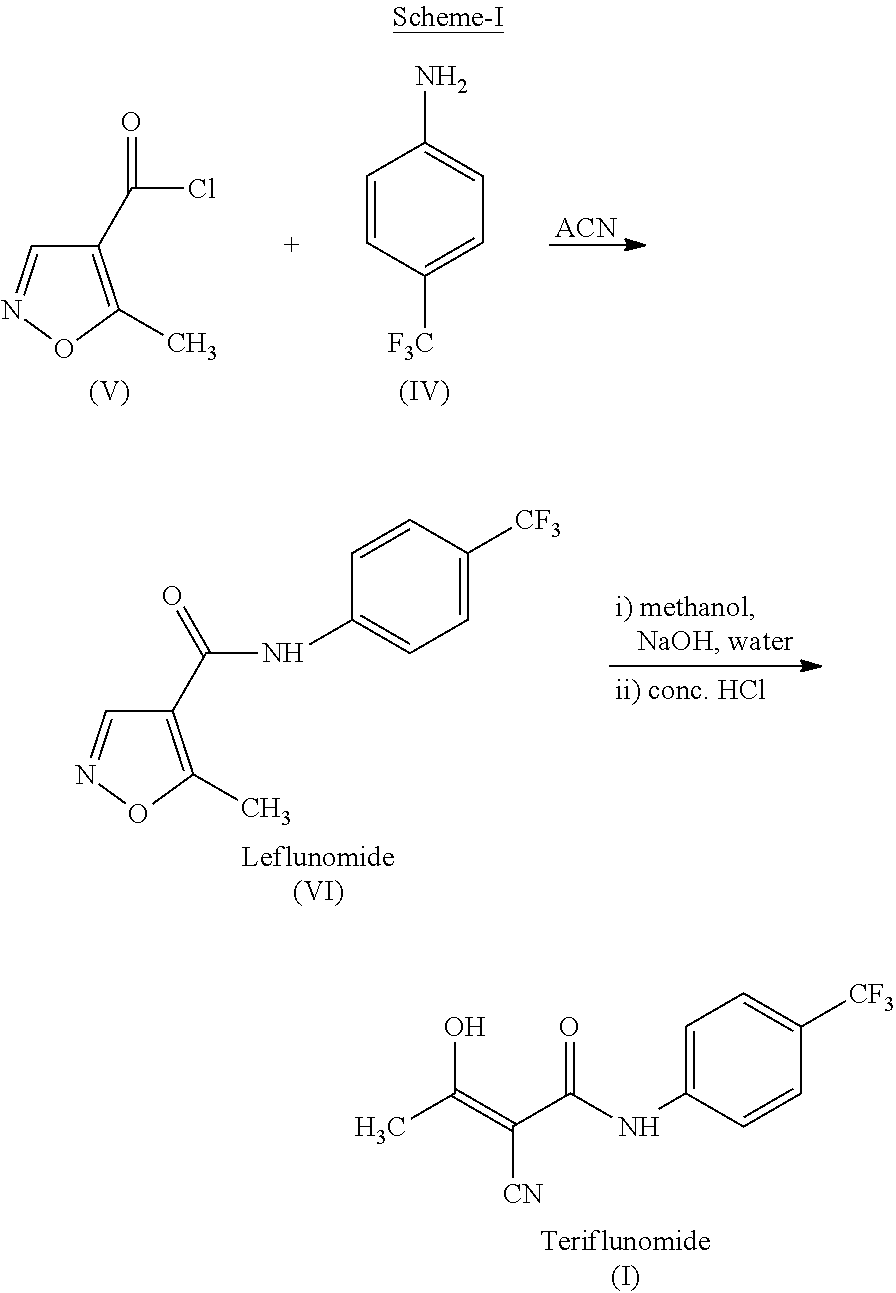
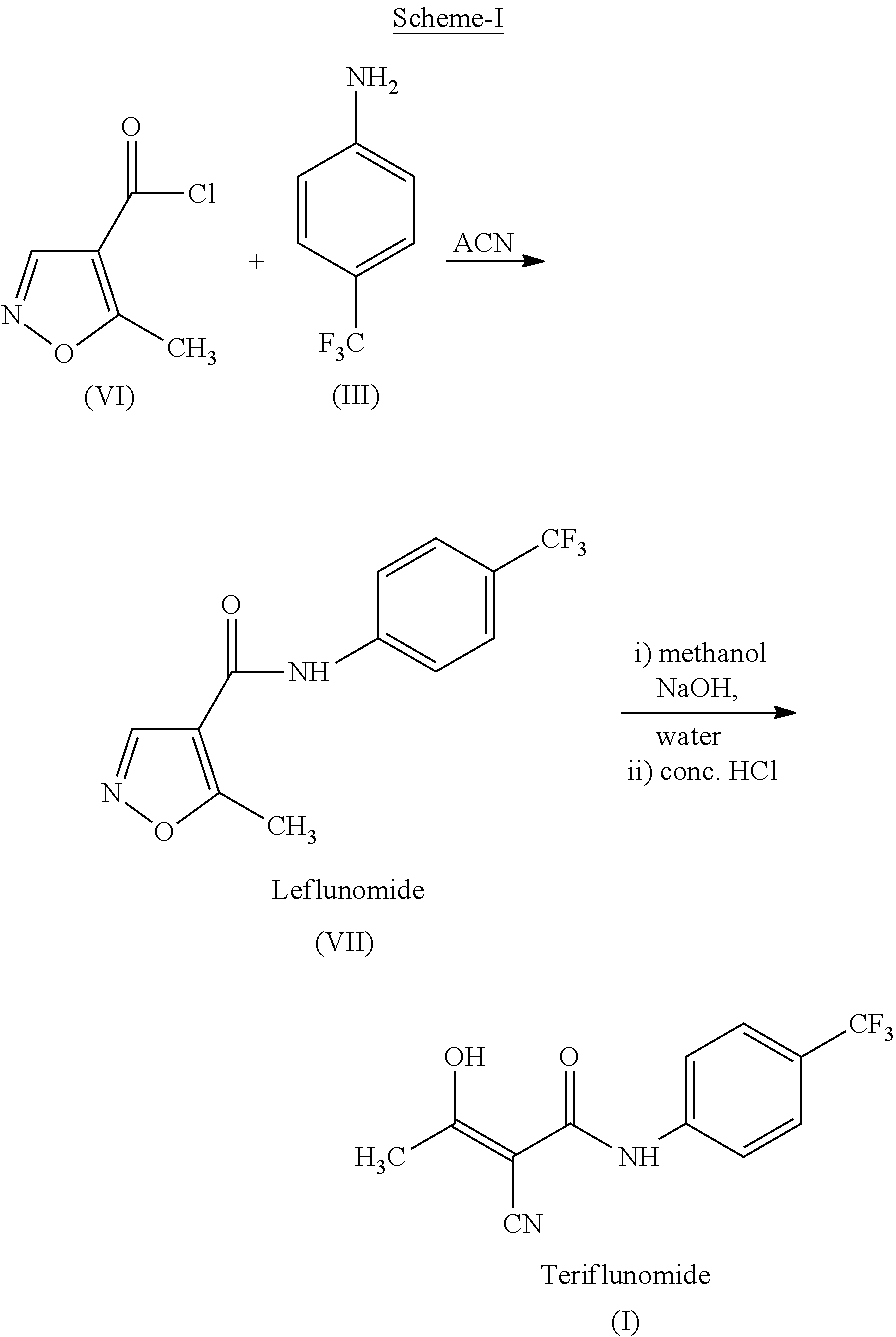
The present invention provides a process for the preparation of Teriflunomide (Formula-I). The present invention describes the synthesis of Teriflunomide without isolating the intermediate Leflunomide. Teriflunomide is prepared from 5-Methyl isoxazole-4-carboxylic acid by converting to its acid chloride and coupling with 4-trifluoromethyl aniline to obtain Leflunomide (which is not isolated) followed by ring opening reaction using aq. Sodium Hydroxide to form Teriflunomide. In other words, the process is telescoped from 5-methylisoxazole-4-carbonyl chloride.
Owner:BIOCON LTD
Use of the combination of teriflunomide and interferon beta for treating multiple sclerosis
Owner:SANOFI AVENTIS US LLC
Application of a kind of teriflunomide in preparation of medicine for preventing foot-and-mouth disease virus infection
ActiveCN109758447BLow cytotoxicityLow toxicityAntiviralsNitrile/isonitrile active ingredientsDiseaseAcupuncture
The invention relates to the application of teriflunomide in the preparation of medicines for preventing foot-and-mouth disease virus infection, and belongs to the technical field of veterinary medicines. The application of the invention can provide a class of highly efficient, safe and quality-controllable anti-foot-and-mouth disease virus medicine for further controlling the spread of foot-and-mouth disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Simple preparation method of teriflunomide
InactiveCN113072464AHigh yieldEasy to recycleCarboxylic acid nitrile preparationOrganic compound preparationLeflunomideCombinatorial chemistry
The invention provides a simple preparation method of teriflunomide, and belongs to the field of medicinal chemistry. The preparation method comprises the following steps of: (1) mixing 5-methylisoxazole-4-formic acid and a condensing agent in a solvent under an alkaline condition, and carrying out condensation reaction to obtain an active ester system; (2) mixing the active ester system and 4-trifluoromethylaniline in a solvent, and carrying out condensation reaction to obtain an intermediate leflunomide; and (3) carrying out alkali treatment and acid treatment on the obtained intermediate leflunomide to obtain teriflunomide. According to the method, the 5-methylisoxazole-4-formic acid reacts with the 4-trifluoromethylaniline in the form of active ester, so that the reaction activity of the 5-methylisoxazole-4-formic acid and the 4-trifluoromethylaniline is improved, the reaction condition is mild, the obtained intermediate leflunomide does not need to be purified, and the yield of teriflunomide is improved.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
A kind of teriflunomide microemulsion, preparation method and application
ActiveCN108324687BPromote transdermal absorptionDissolution rate is fastAntipyreticAnalgesicsHigh concentrationPharmaceutical drug
The invention discloses a teriflunomide microemulsion, a preparation method and application. The preparation method includes: well mixing an emulsifier and a co-emulsifier, then adding an oil phase; dissolving teriflunomide into deionized water to obtain a teriflunomide solution aqueous phase; in the process of stirring, dropwise adding a teriflunomide solution into a mixed oil phase to form a transparent drug-containing microemulsion, wherein a proportion of the oil phase, the aqueous phase, the emulsifier and the co-emulsifier is optimized according to a pseudo-ternary phase diagram. Percutaneous administration, the microemulsion can improve solubleness of slightly soluble drug, form high concentration gradient and promote drug absorption; a hydrophobic portion of the microemulsion interacts with cuticle to disturb lipid bilayer structure, a hydrophilic portion can hydrate the cuticle, combined action of the hydrophobic portion and the hydrophilic portion can enhance permeability ofthe drug on the cuticle, and permeation and absorption of the drug are facilitated. A new dosage form is provided for clinic application of teriflunomide.
Owner:ANHUI MEDICAL UNIV
Environmental-protection simple preparation method of teriflunomide
ActiveCN105272882AReduce usageShort reaction stepsCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventLeflunomide
The invention discloses an environmental-protection simple preparation method of teriflunomide; leflunomide is used as a starting material, a heterogeneous reaction is adopted, an organic solvent is avoided from being used in a synthesis reaction process, and thus the high-purity teriflunomide can be prepared.
Owner:CINKATE PHARMA INTERMEDIATES
Preparation method of teriflunomide and intermediate thereof
ActiveCN103848756BHigh yieldReduce manufacturing costCarboxylic acid nitrile preparationOrganic compound preparationElectrophilic additionIsomerization
The invention belongs to the technical field of medicinal chemistry and discloses a preparation method of teriflunomide and an intermediate thereof. The preparation method of the teriflunomide provided by the invention comprises the following steps: getting a compound with a structure as shown in formula I and an azide in an organic solvent to generate nucleophilic substitution reaction to generate a compound with the structure as shown in formula II; getting and mixing the compound with the structure as shown in the formula II with the organic solvent, heating to obtain the compound with the structure as shown in formula III; under inert-gas protection and alkaline conditions, getting the compound with the structure as shown in the formula III and the compound with the structure as shown in the formula IV to generate electrophilic addition and isomerization reaction in the organic solvent to obtain the teriflunomide. According to the preparation method of the teriflunomide provided by the invention, the reaction intermediate is not needed to be purified, operation is simple, yield of the teriflunomide is improved, production cost of the teriflunomide is lowered, and therefore, the preparation method is more beneficial to industrial production of the teriflunomide.
Owner:HYBIO PHARMA WUHAN CO LTD
Continuous flow teriflunomide preparation process
InactiveCN113666842ASolve the impact of damageAdequate responseCarboxylic acid nitrile preparationOrganic compound preparationAcetyl chlorideBiochemical engineering
The invention discloses a continuous flow teriflunomide preparation process which comprises the following steps: using a continuous flow reactor, taking cyanoacetic acid as a starting material, preparing cyanoacetyl chloride through chlorination, synthesizing a teriflunomide intermediate through the cyanoacetyl chloride and p-trifluoromethylaniline, and synthesizing teriflunomide through the intermediate and acetyl chloride. The method has the advantages of high safety, low cost, low energy consumption and high production yield.
Owner:河北凯威恒诚制药有限公司
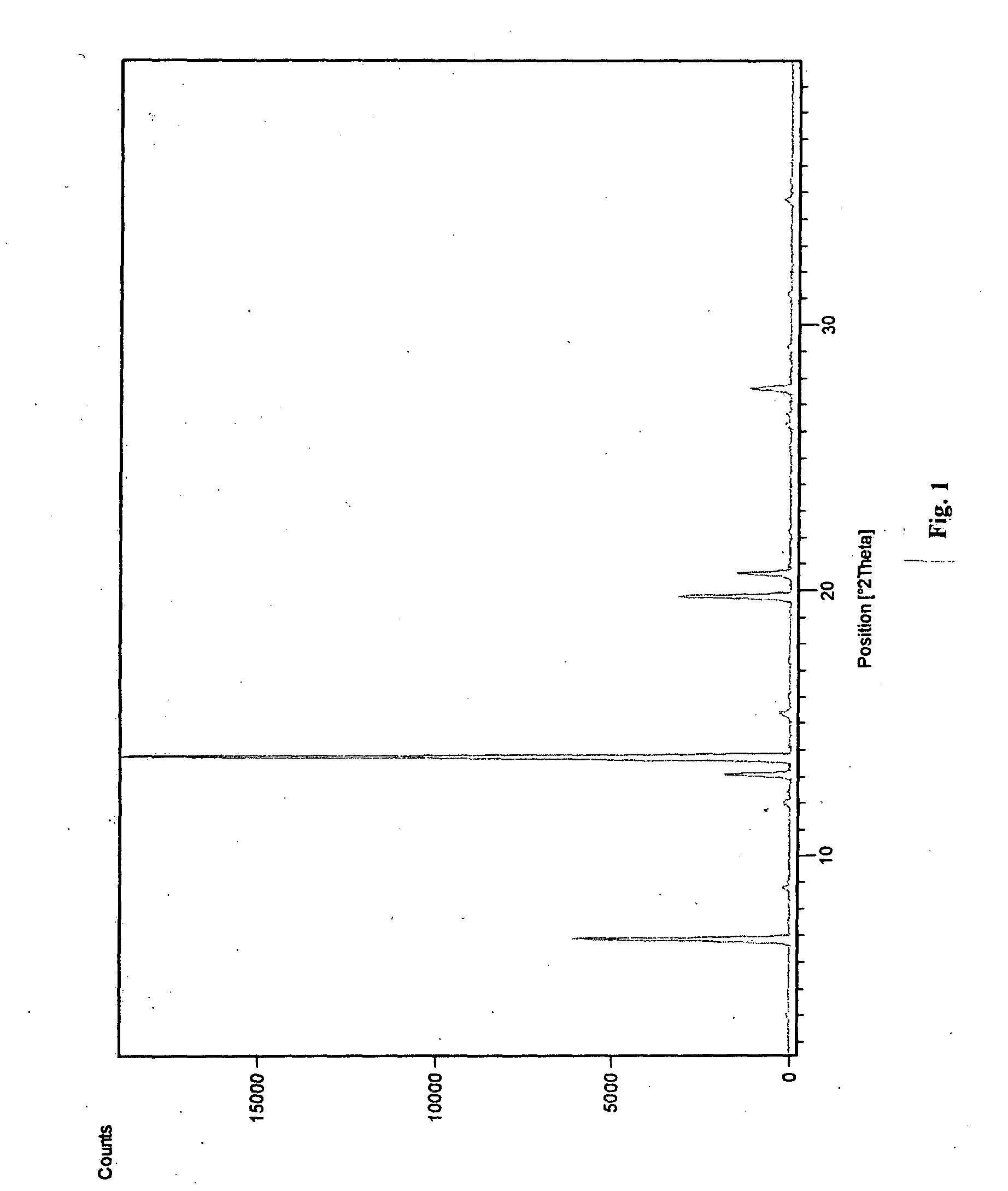
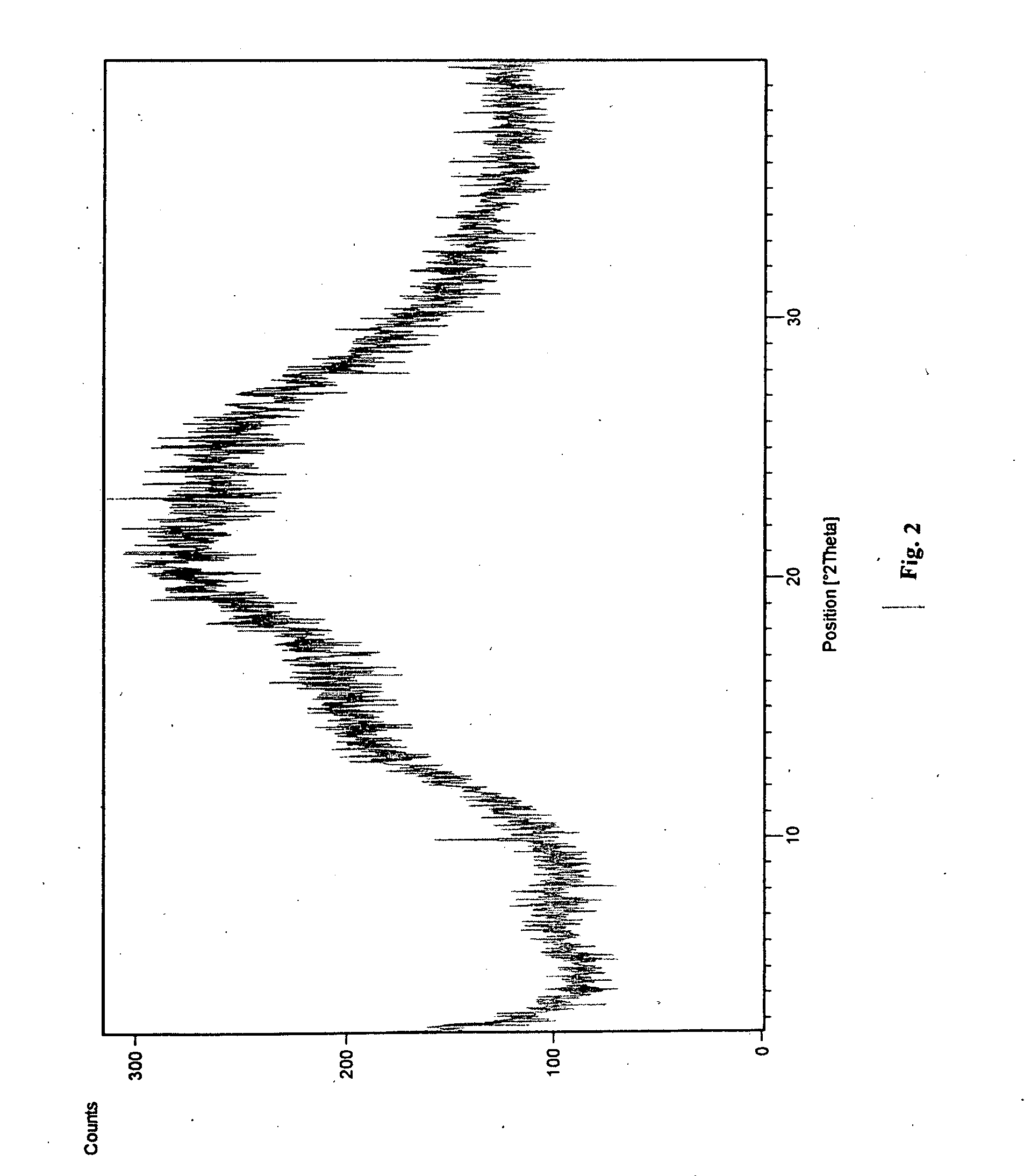
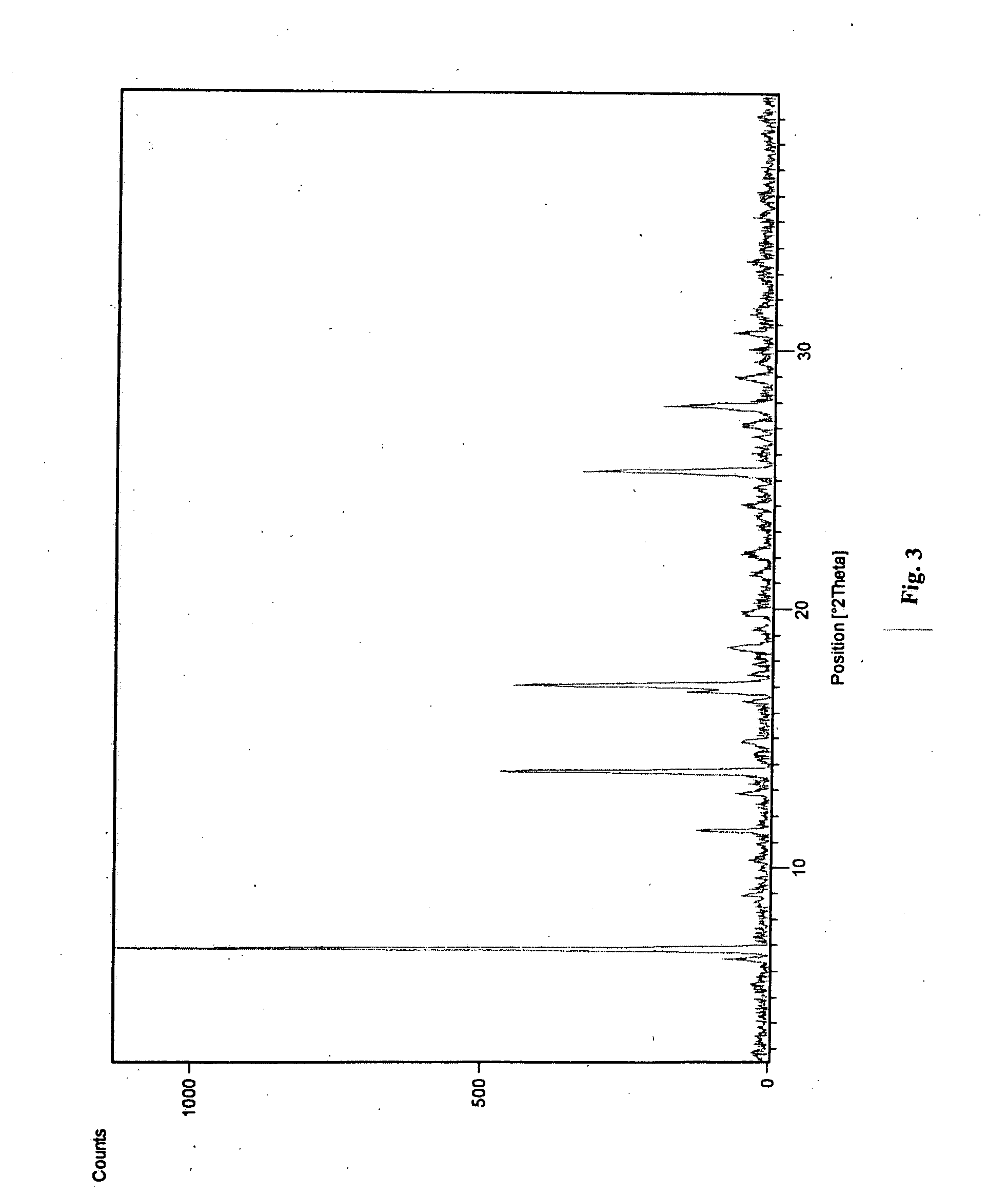
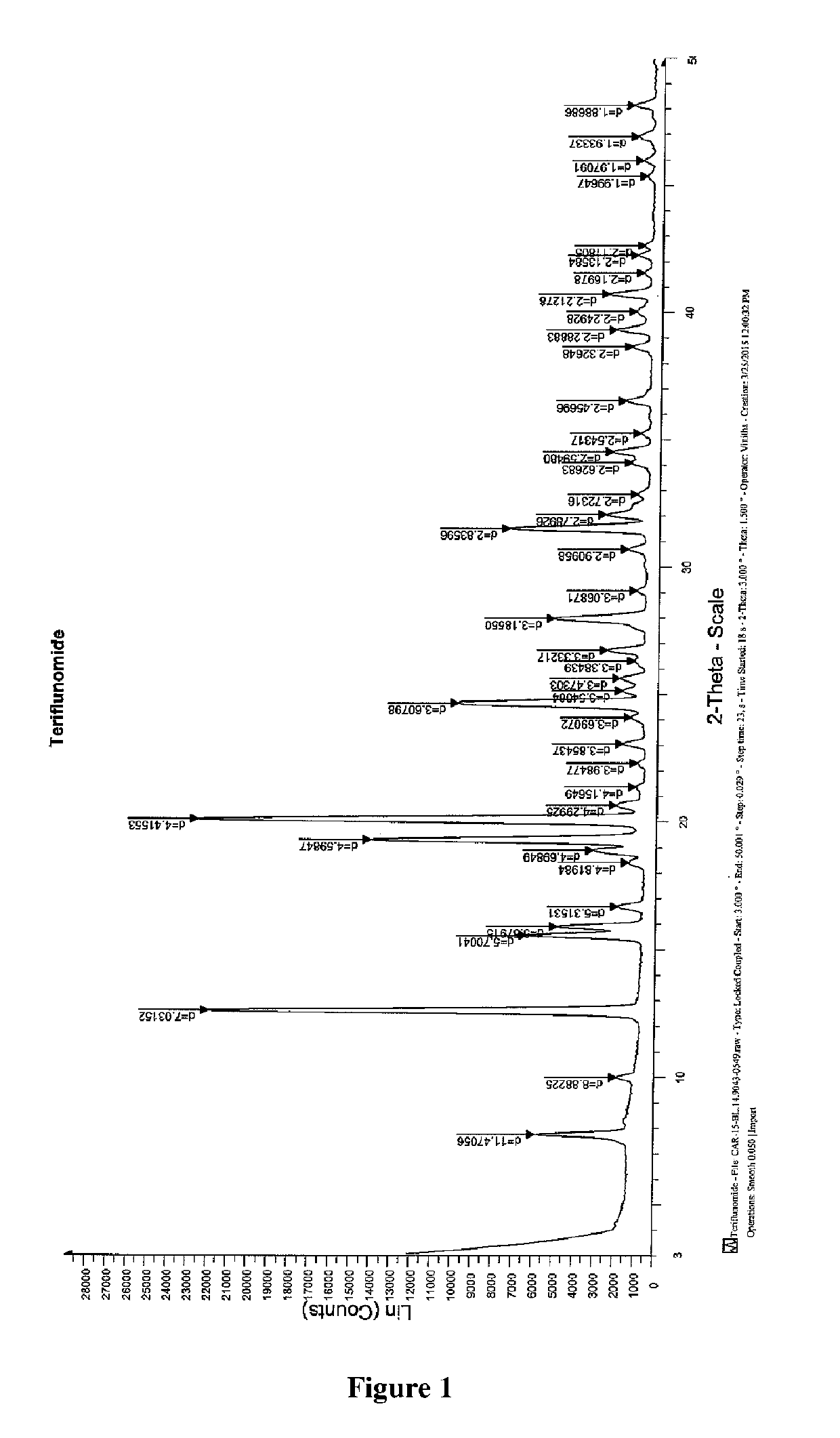
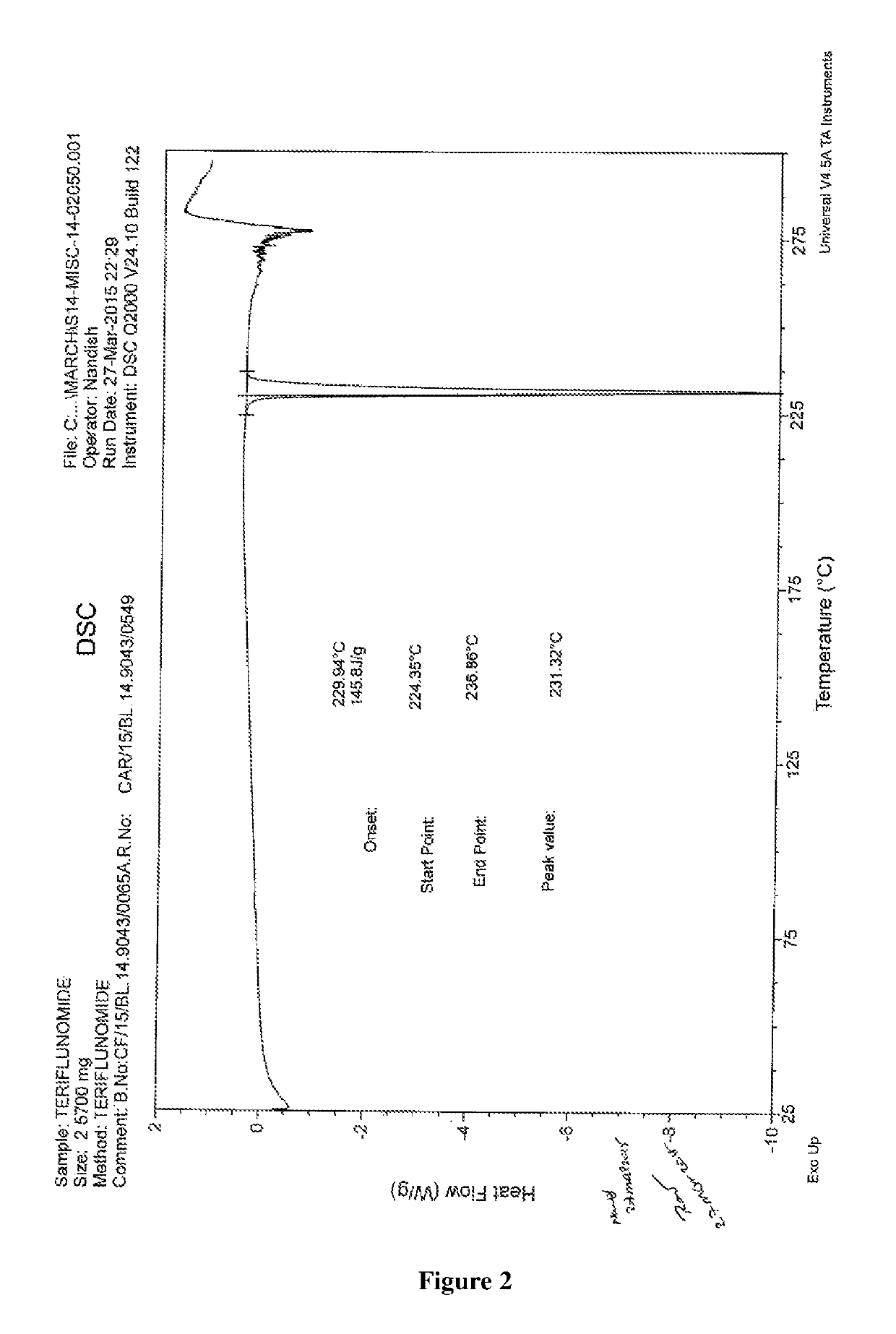
Novel polymorphic form of teriflunomide salts
The present invention provides a new polymorph Form I of Teriflunomide sodium and a process for preparation thereof. The present invention provides an amorphous form of Teriflunomide sodium and a process for preparation thereof. The present invention provides a new polymorph Form I of Teriflunomide potassium and a process for preparation thereof. The present invention provides an amorphous form of Teriflunomide potassium and a process for preparation thereof. The present invention also provides particle size of Teriflunomide and its salts.
Owner:ALEMBIC PHARMA
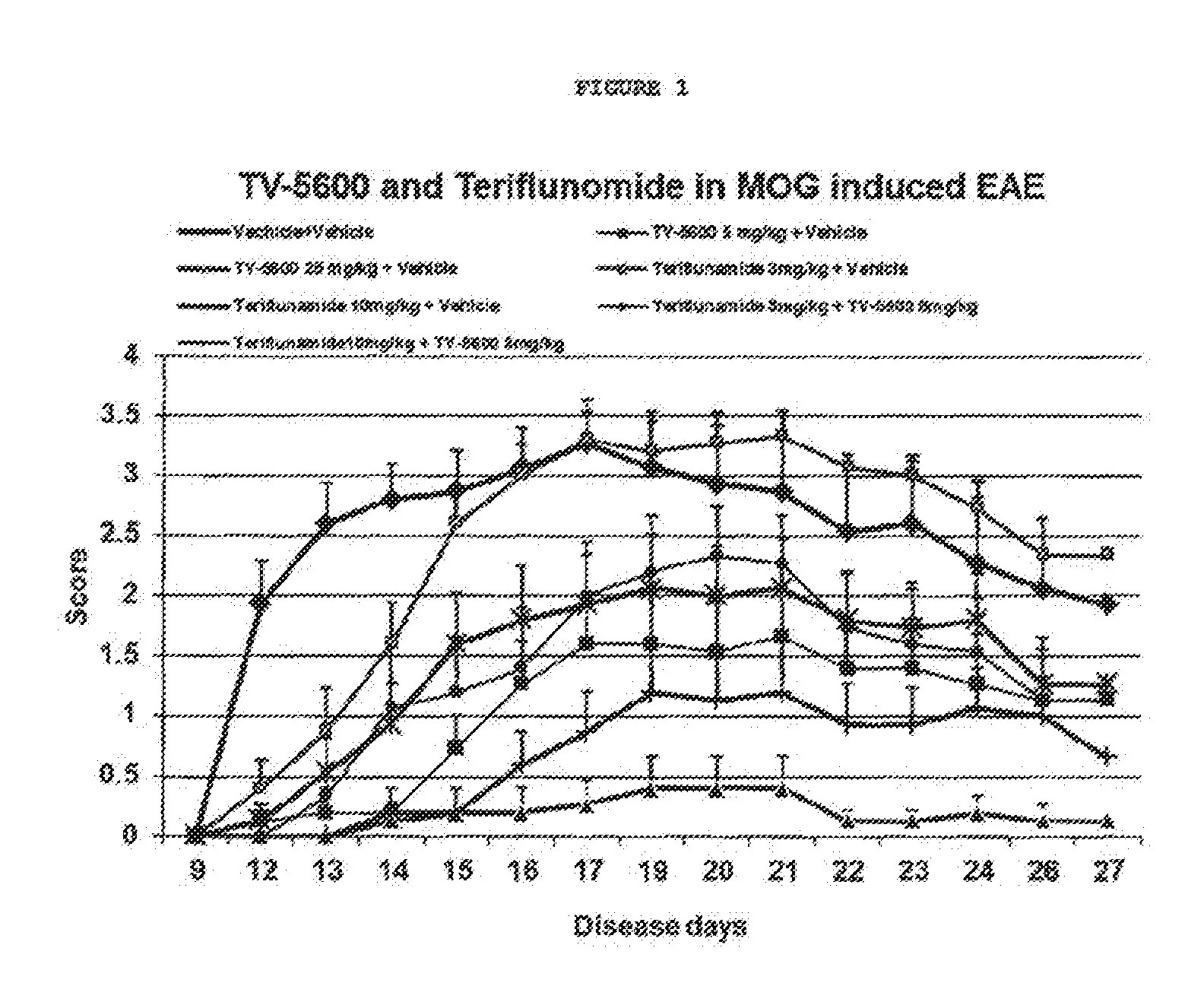
Treatment of multiple sclerosis with combination of laquinimod and teriflunomide
InactiveUS20160317525A1Useful in treatmentNervous disorderPharmaceutical delivery mechanismLaquinimodMinimal effective dose
This invention provides a method of treating a subject afflicted with multiple sclerosis (MS) or presenting a clinically isolated syndrome (CIS) comprising administering to the subject laquinimod as an add-on therapy to or in combination with a greater than minimal effective dose of teriflunomide. This invention also provides a package and a pharmaceutical composition comprising laquinimod and a greater than minimal effective dose of teriflunomide for treating a subject afflicted with MS or presenting a CIS. This invention also provides laquinimod for use as an add-on therapy or in combination with a greater than minimal effective dose of teriflunomide in treating a subject afflicted with MS or presenting a CIS. This invention further provides use of laquinimod and a greater than minimal effective dose of teriflunomide in the preparation of a combination for treating a subject afflicted with MS or presenting a CIS.
Owner:TEVA PHARMA IND LTD
Process for preparing teriflunomide
InactiveUS8389757B2Easy to handleCarboxylic acid nitrile preparationOrganic compound preparationPolymer scienceTeriflunomide
Owner:ALEMBIC LTD
Process for the preparation of teriflunomide
ActiveUS10526279B2High purityHigh yieldOrganic compound preparationMaterial heat developmentCarboxylic acidAniline
The present invention provides a process for the preparation of Teriflunomide (Formula-I). The present invention describes the synthesis of Teriflunomide without isolating the intermediate Leflunomide. Teriflunomide is prepared from 5-Methyl isoxazole-4-carboxylic acid by converting to its acid chloride and coupling with 4-trifluoromethyl aniline to obtain Leflunomide (which is not isolated) followed by ring opening reaction using aq. Sodium Hydroxide to form Teriflunomide. In other words, the process is telescoped from 5-methylisoxazole-4-carbonyl chloride.
Owner:BIOCON LTD
Process for preparation of teriflunomide
InactiveUS8420856B2Easy to handleOrganic compound preparationPreparation by cyanide reactionPolymer scienceTeriflunomide
Owner:ALEMBIC LTD
Combination therapy for treatment of multiple sclerosis
InactiveUS20190091190A1Good effectShow an Increase in severe adverse eventsNervous disorderAntipyreticSide effectOral treatment
The present invention relates to a method of treating MS in a human patient in need of such treatment and comprises administering to said patient a combination therapy in a single oral dosage form (e.g. a tablet or capsule) of dim ethyifurrta rate and one agent selected from teriflunomide (or its prodrug leflunomide), fingolimod and laquinimod. This combination is more effective than the single agents alone and / or has reduced side effects and better tolerability than the single agents alone and / or can be given in a reduced frequency. Moreover, the present invention is directed to a pharmaceutical composition suitable for the oral treatment of multiple sclerosis consisting of dimethylfumarate and one agent selected from teriflunomide, fingolimod and laquinimod as active ingredients and one or more pharmaceutically acceptable excipients.
Owner:BIOGEN SWISS MFG GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com