Application of sodium teriflunomide to preparation of medicine for treating autoimmune diseases
A technology for sodium tirimide and autoimmune diseases, which is applied in the field of sodium salt of the active metabolite of leflunomide, which can solve the problems of autoimmune diseases such as lupus erythematosus without tirimide sodium, and avoid the first-pass effect , Large exposure in vivo, high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Preparation of Terimide Sodium
[0073] 1.1 Preparation of Terimide
[0074] Leflunomide is dissolved in alkali solution for hydrolysis reaction, acidified to obtain Terimide, and filtered to obtain Terimide wet product. This wet product directly enters the next step of reaction.
[0075] 1.2 Preparation of Terimide Sodium
[0076] The wet product of Terimide was put into aqueous sodium hydroxide solution, and the temperature was raised to react for 2 hours, the insoluble matter was filtered off, the filtrate was lowered to room temperature to grow crystals, and the crude product of Terimide Sodium was filtered and dried. After recrystallization from ethanol, pure product Terimide Sodium (purity ≥ 99.5) was obtained.
Embodiment 2
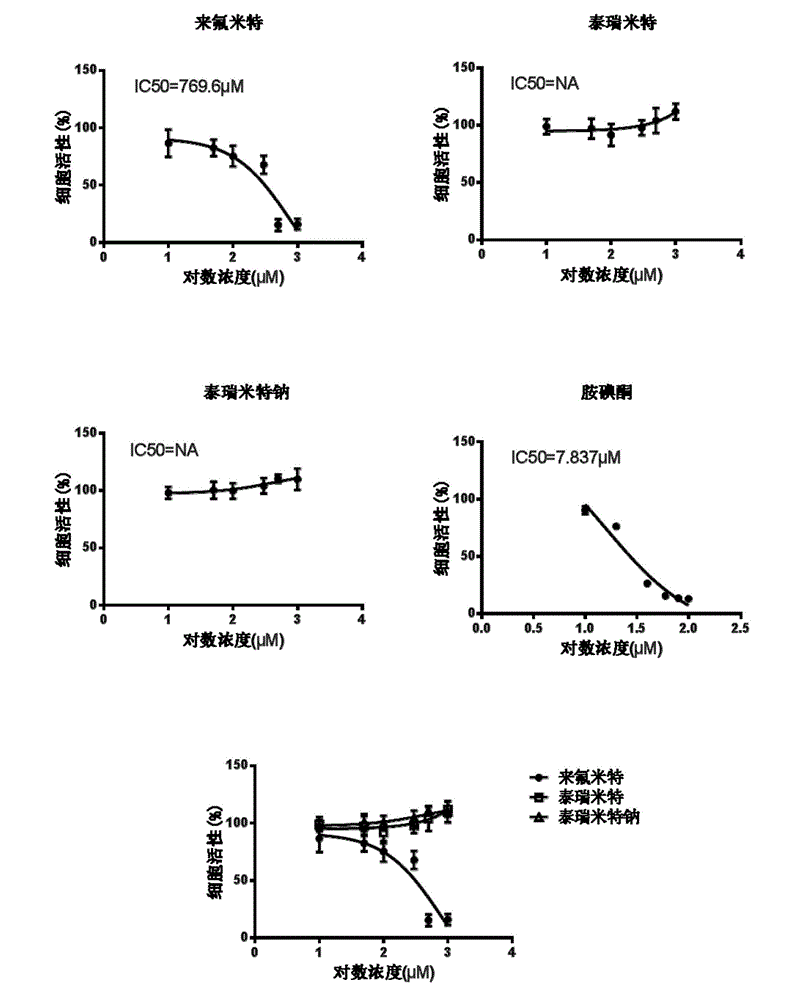
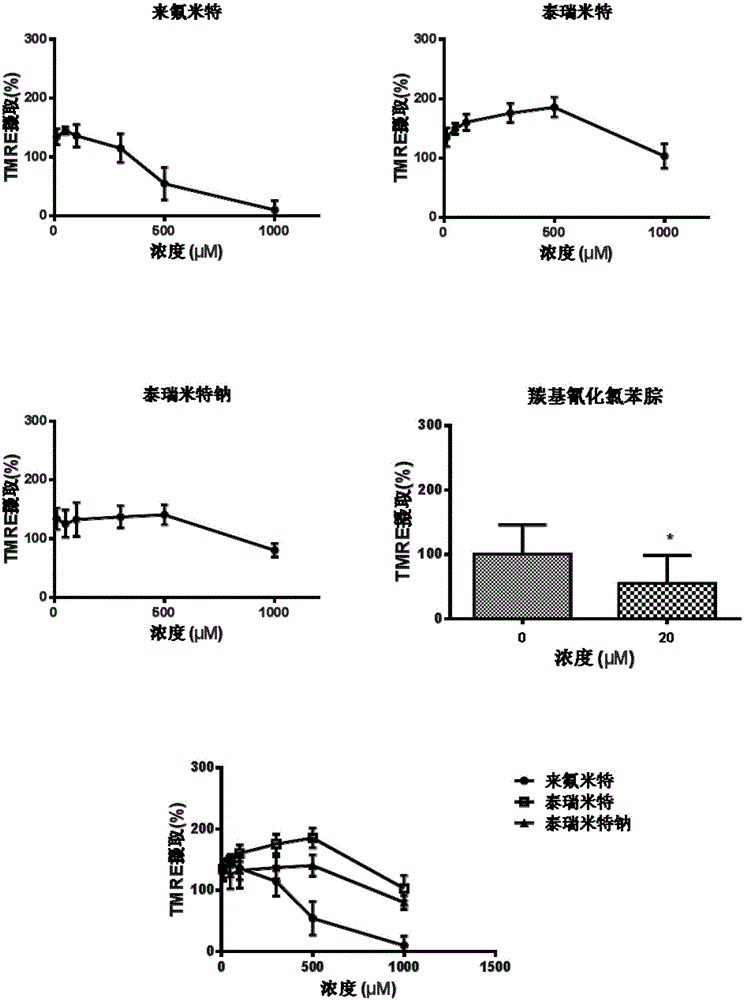
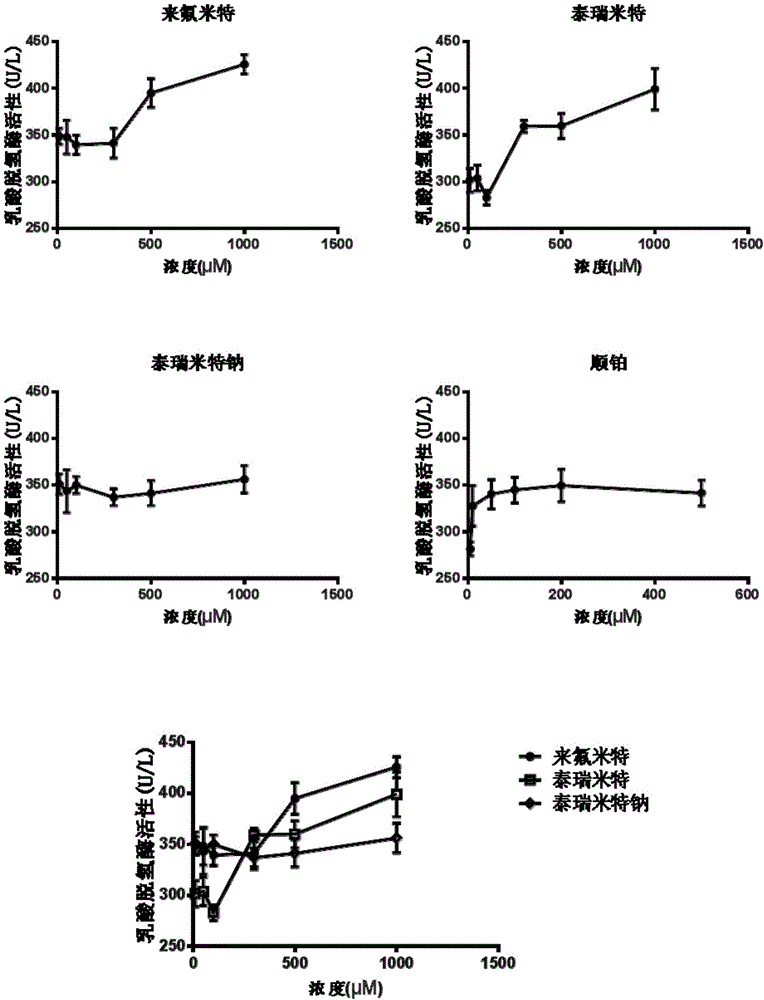
[0078] Safety evaluation of tirimide sodium in primary human hepatocytes
[0079] The purpose of this experiment is to detect the toxicity of tirimide sodium to human liver cells, and compare its toxicity with leflunomide and tirimide, including cell viability test (MTT), lactate dehydrogenase ( LDH) release experiment and mitochondrial membrane potential experiment.
[0080] Experimental steps:
[0081] Rat primary hepatocytes and human primary hepatocytes were used in this experiment. Among them, the primary rat hepatocytes are derived from SD rats, and the isolation of primary hepatocytes has been approved by IACUC2013-06-PGY-03 of the Institute of Medicine. Primary human hepatocytes were purchased from Yingwei Jieji (Shanghai) Trading Co., Ltd., batch number HU4239. The test products are: Terimide, Terimide Sodium and Leflunomide.
[0082] Rat primary hepatocytes were cultured for 3 hours in WilliamsEMedium medium containing 1% penicillin, streptomycin, 1% glutamine, 0.1...
Embodiment 3
[0103] Observation of curative effect of tirimide sodium in spontaneous lupus erythematosus model in female MRL / lpr mice
[0104] The purpose of this study is to observe the therapeutic effect of different doses of the test compound tirimide sodium on lupus model animals, and compare it with glucocorticoids and leflunomide. The animal model used in this study is the MRL / lpr mouse, which has autoreactive CD4 due to the deletion of the Fas gene. + T cells cannot be cleared through the apoptotic pathway, and then produce autoimmune disease symptoms similar to human SLE. In the field of international pharmacology research, it is widely used in the study of the pathogenesis of SLE and the study of drug pharmacodynamics. Considering that female MRL / lpr mice usually start to show symptoms of SLE between the age of 8 weeks and 12 weeks, drug intervention was given to the model mice at the age of 10-11 weeks for 8 consecutive weeks.
[0105] Test design:
[0106] grouping:
[0107] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com