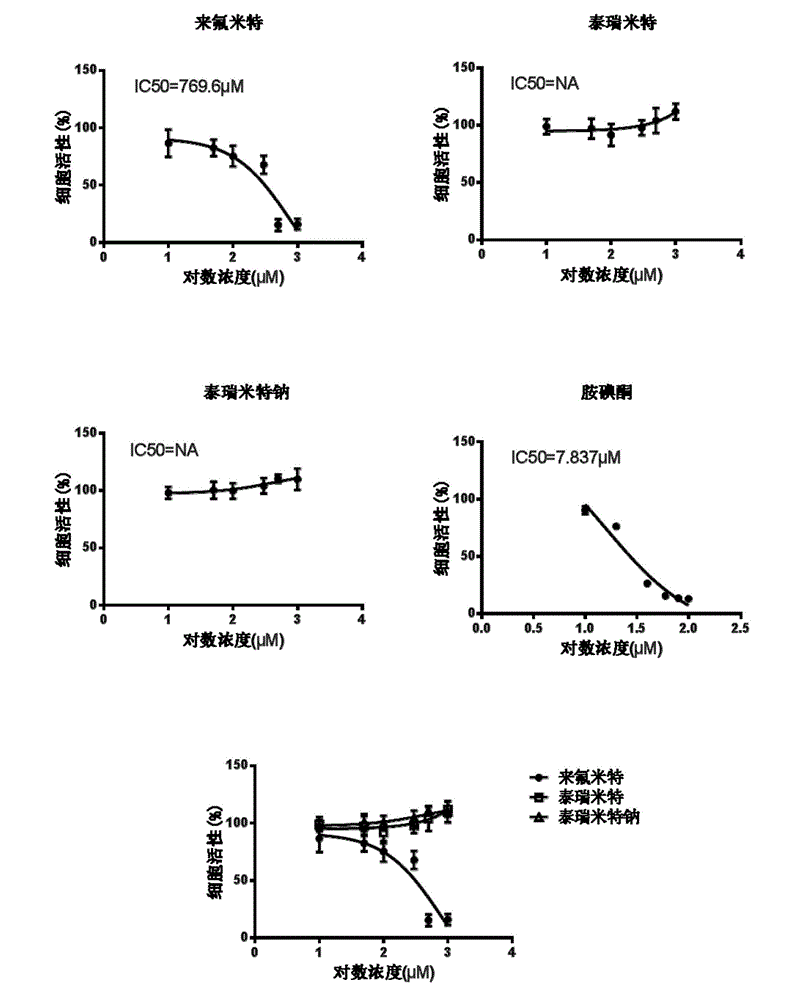
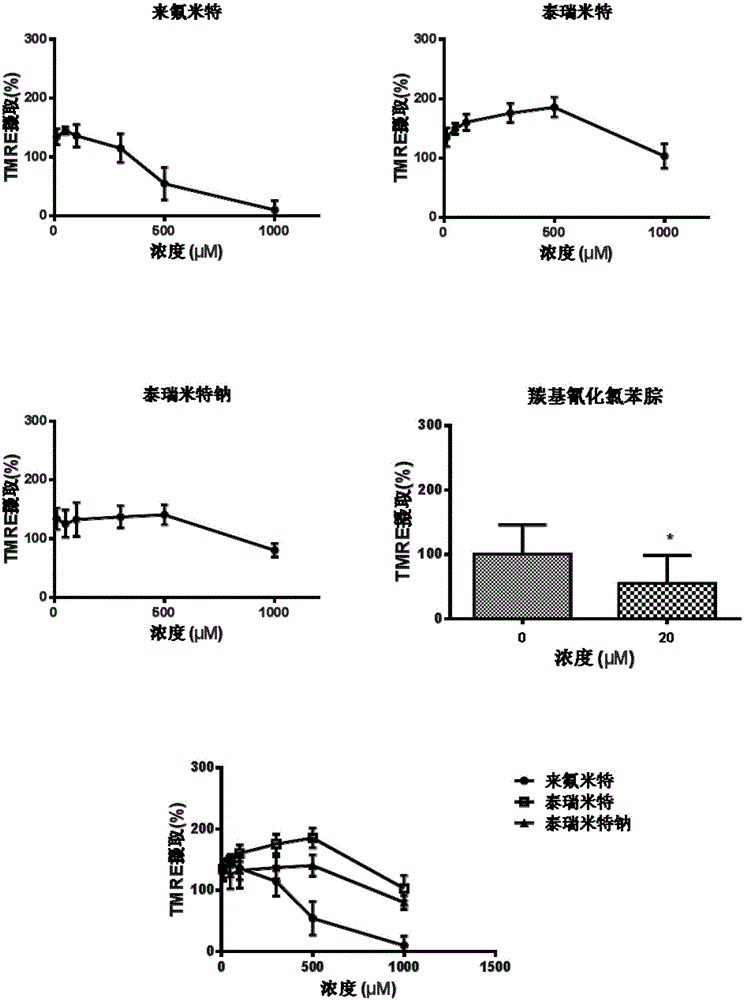
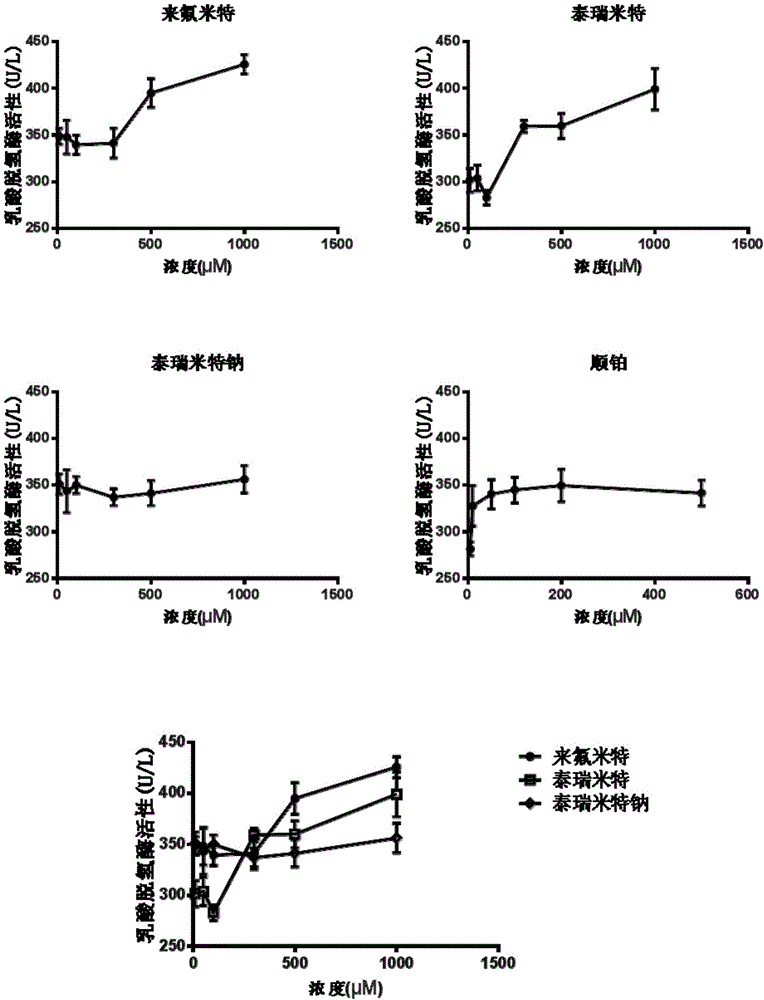
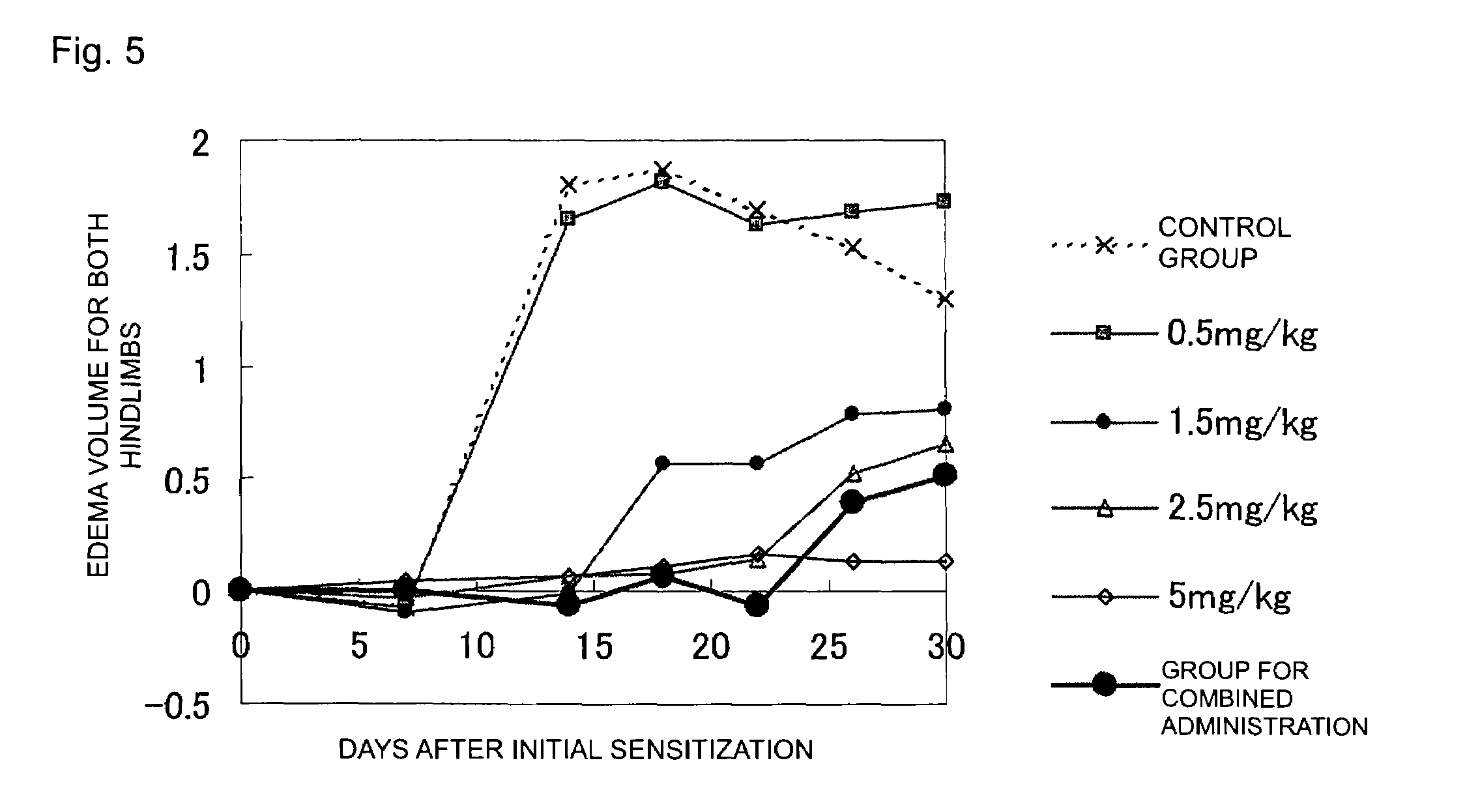
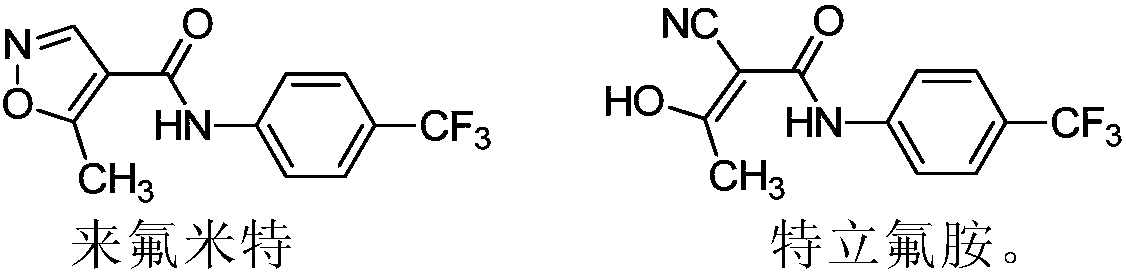
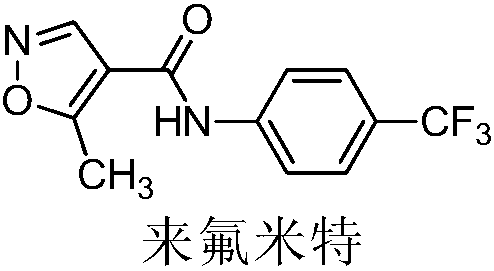
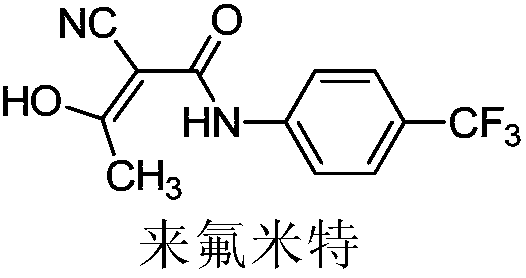
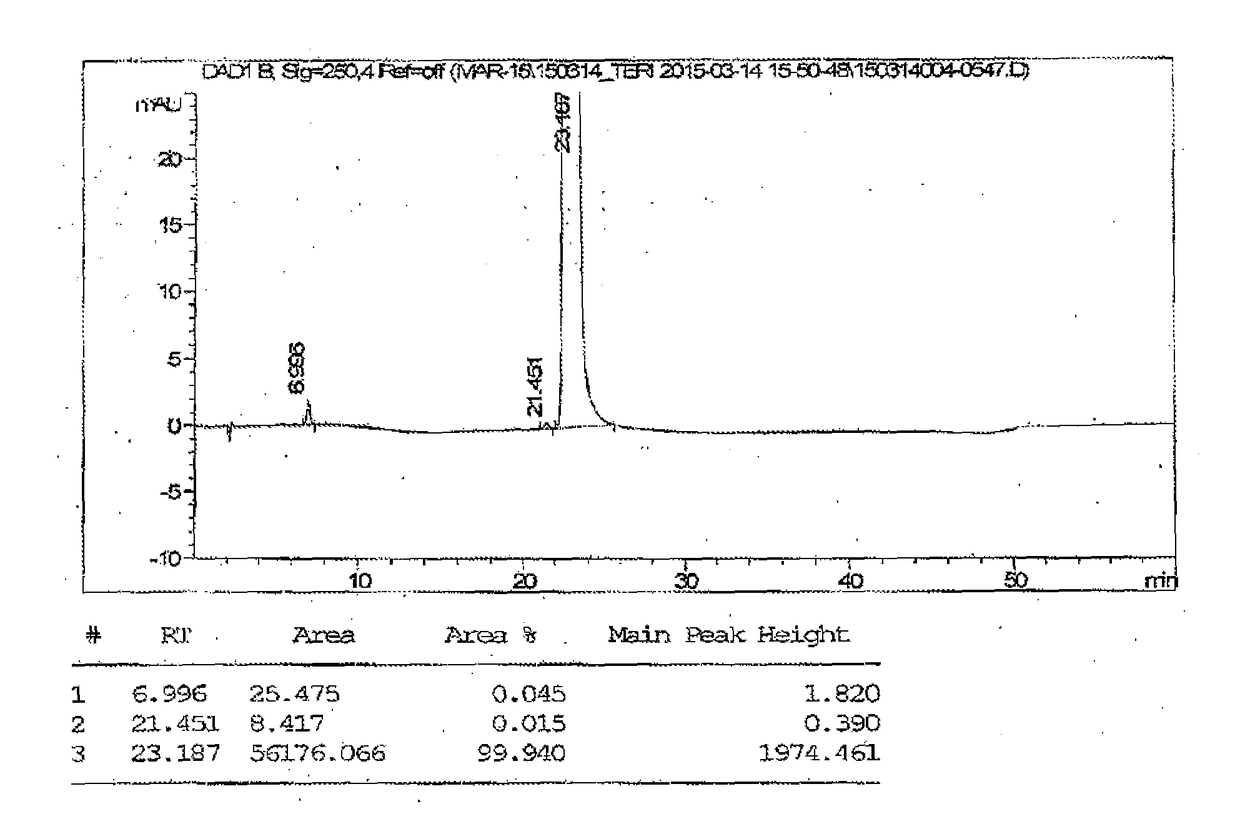
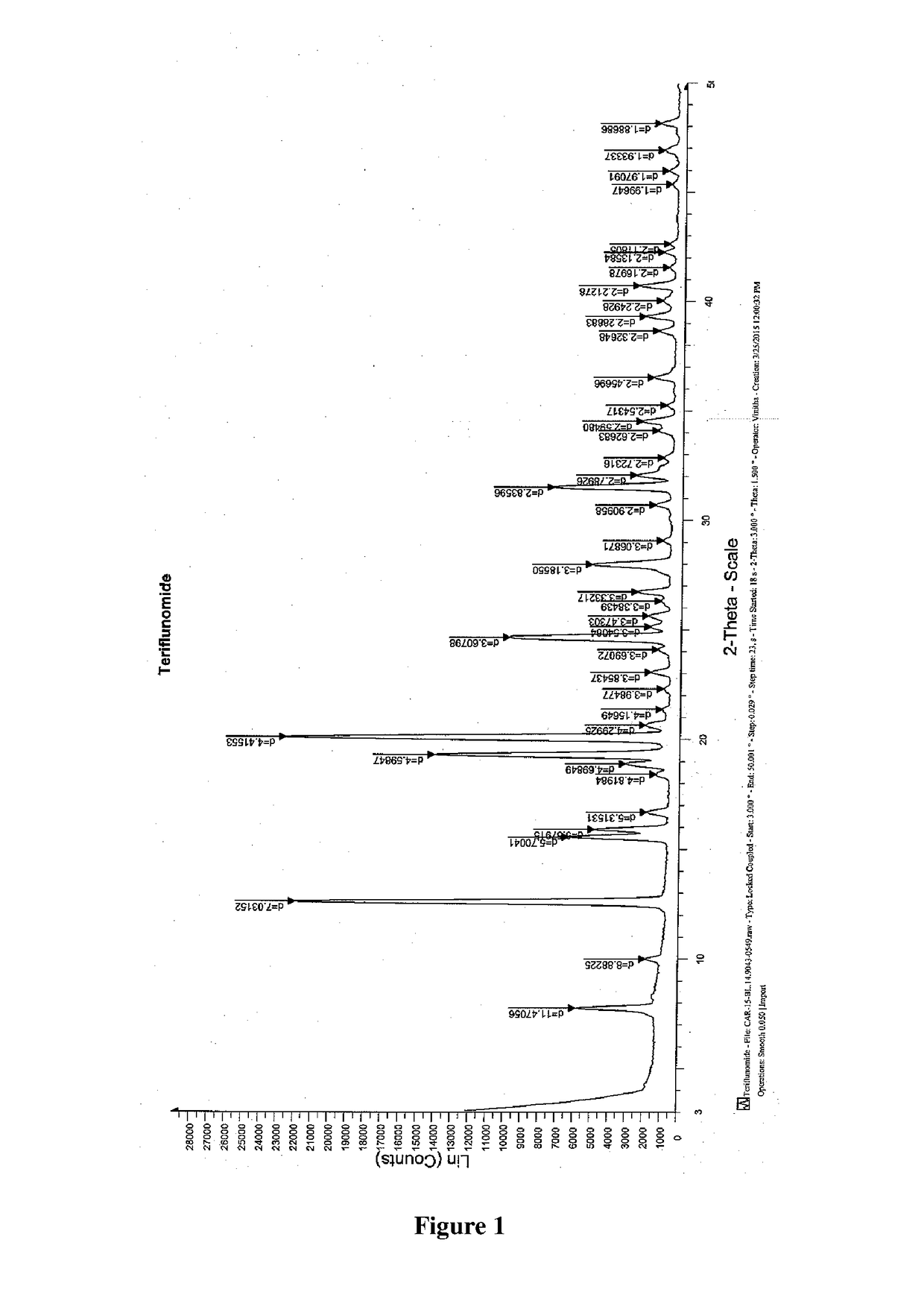
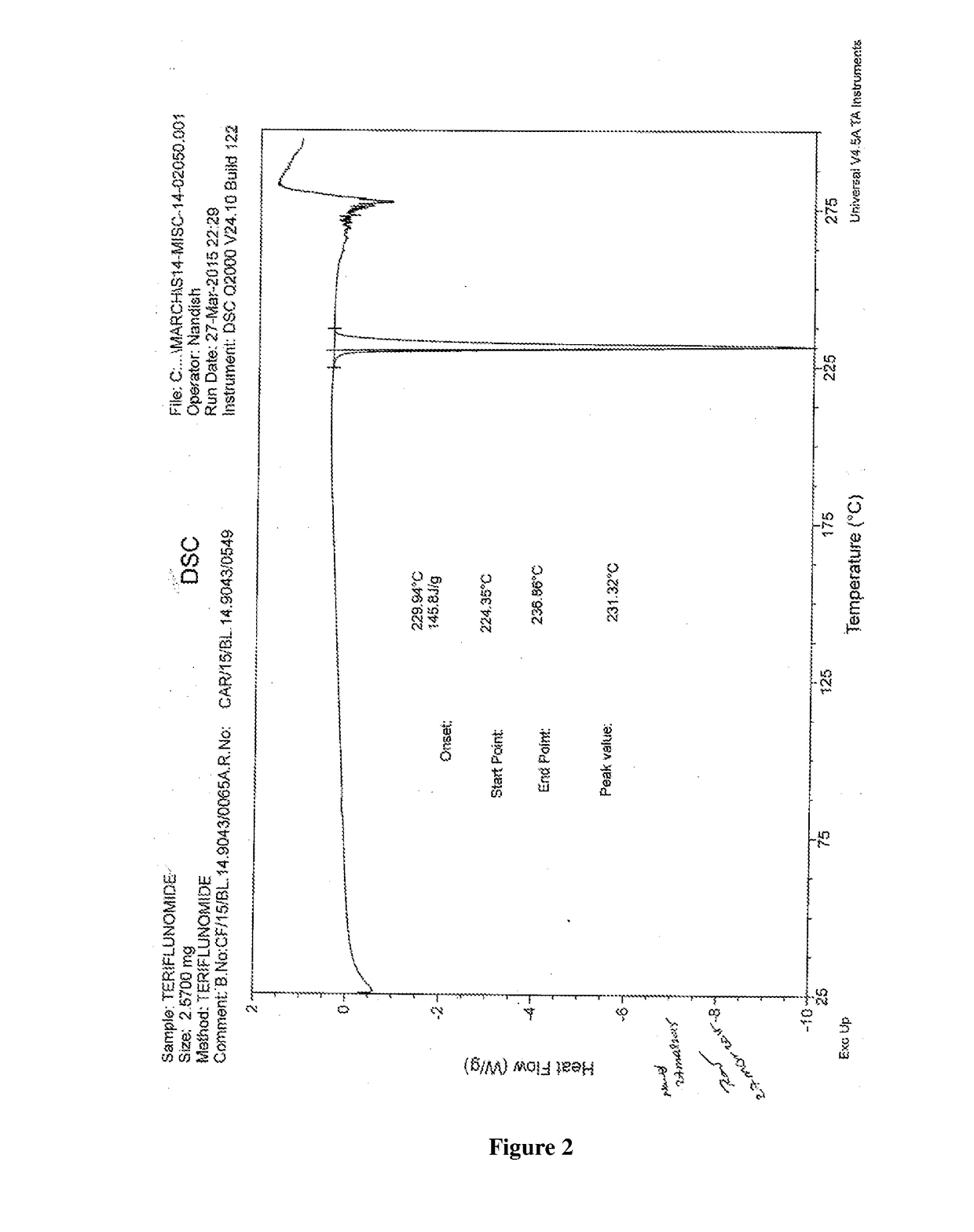
Patents
Literature
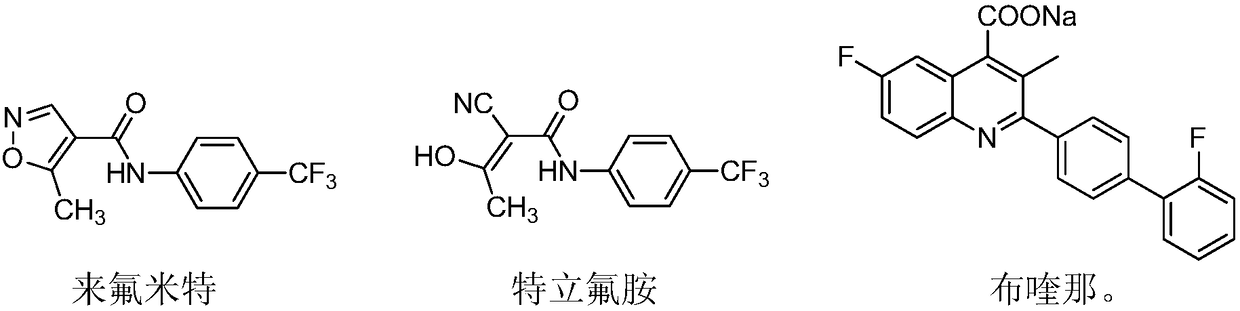
46 results about "Leflunomide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat rheumatoid arthritis, a condition in which the body's defense system (immune system) fails to recognize the body as itself and attacks the healthy tissues around the joints.
Novel external agent
InactiveUS20050158371A1Improve stabilityGood treatment effectBiocideOrganic non-active ingredientsExternal applicationDissolution
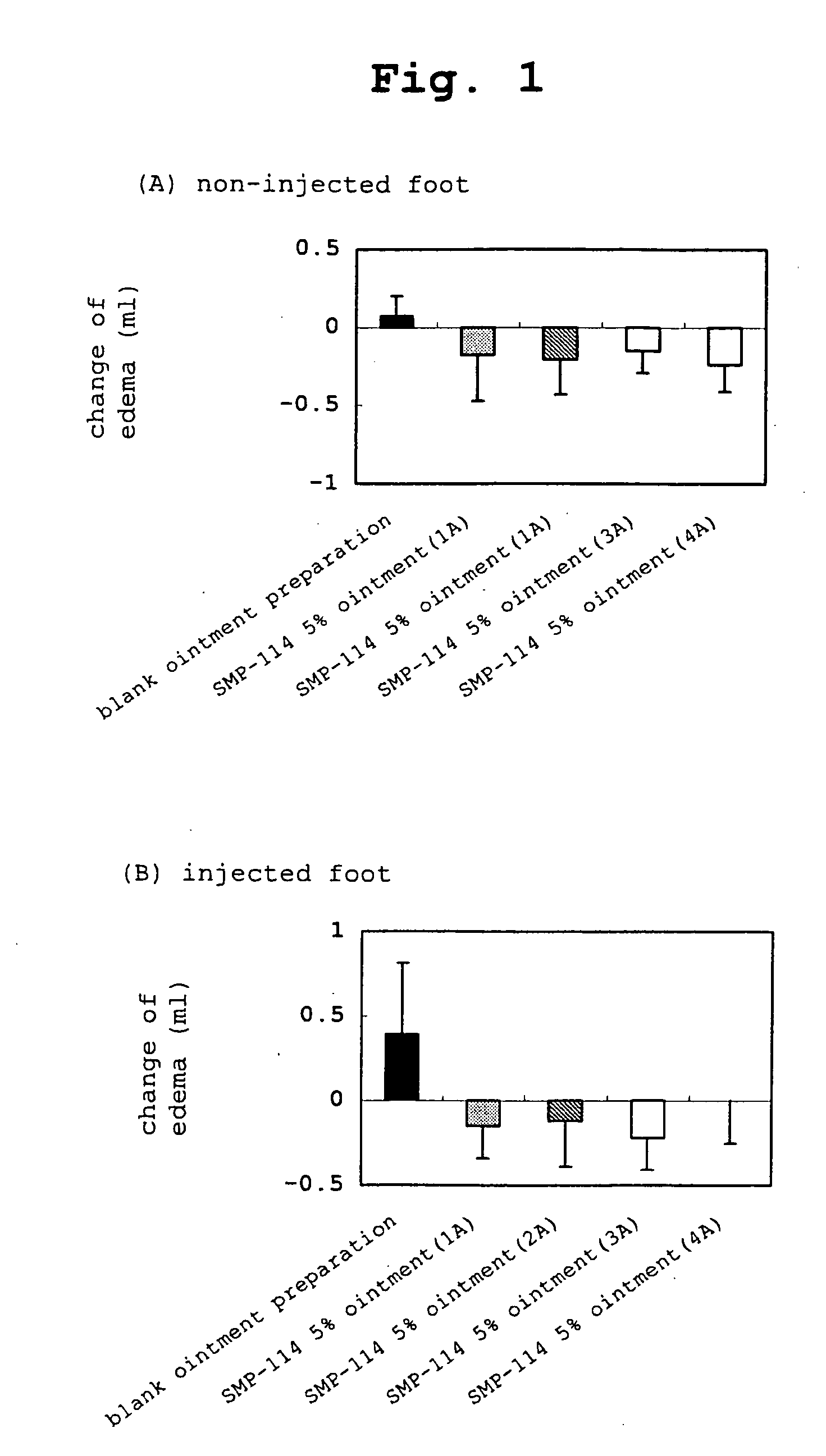
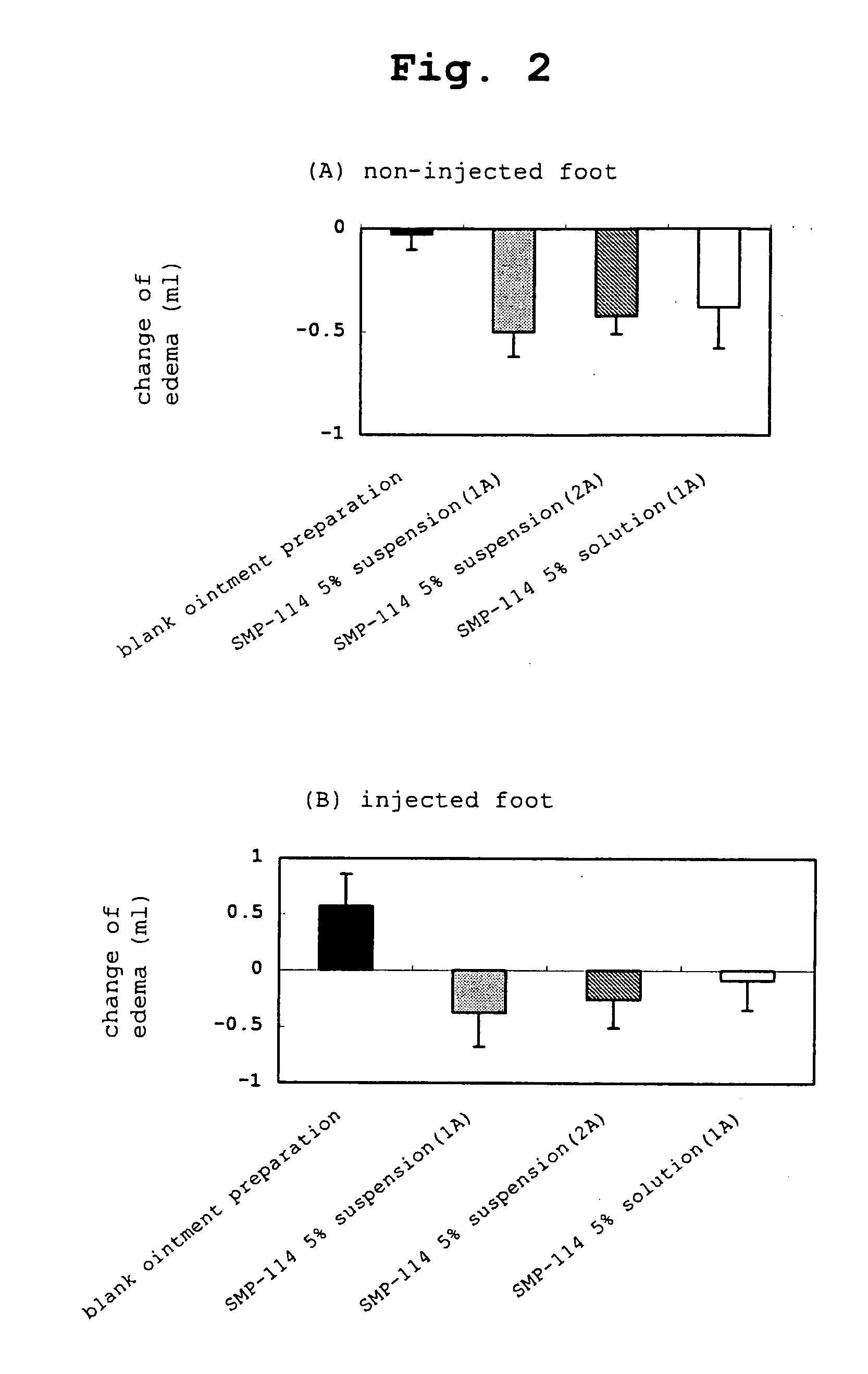
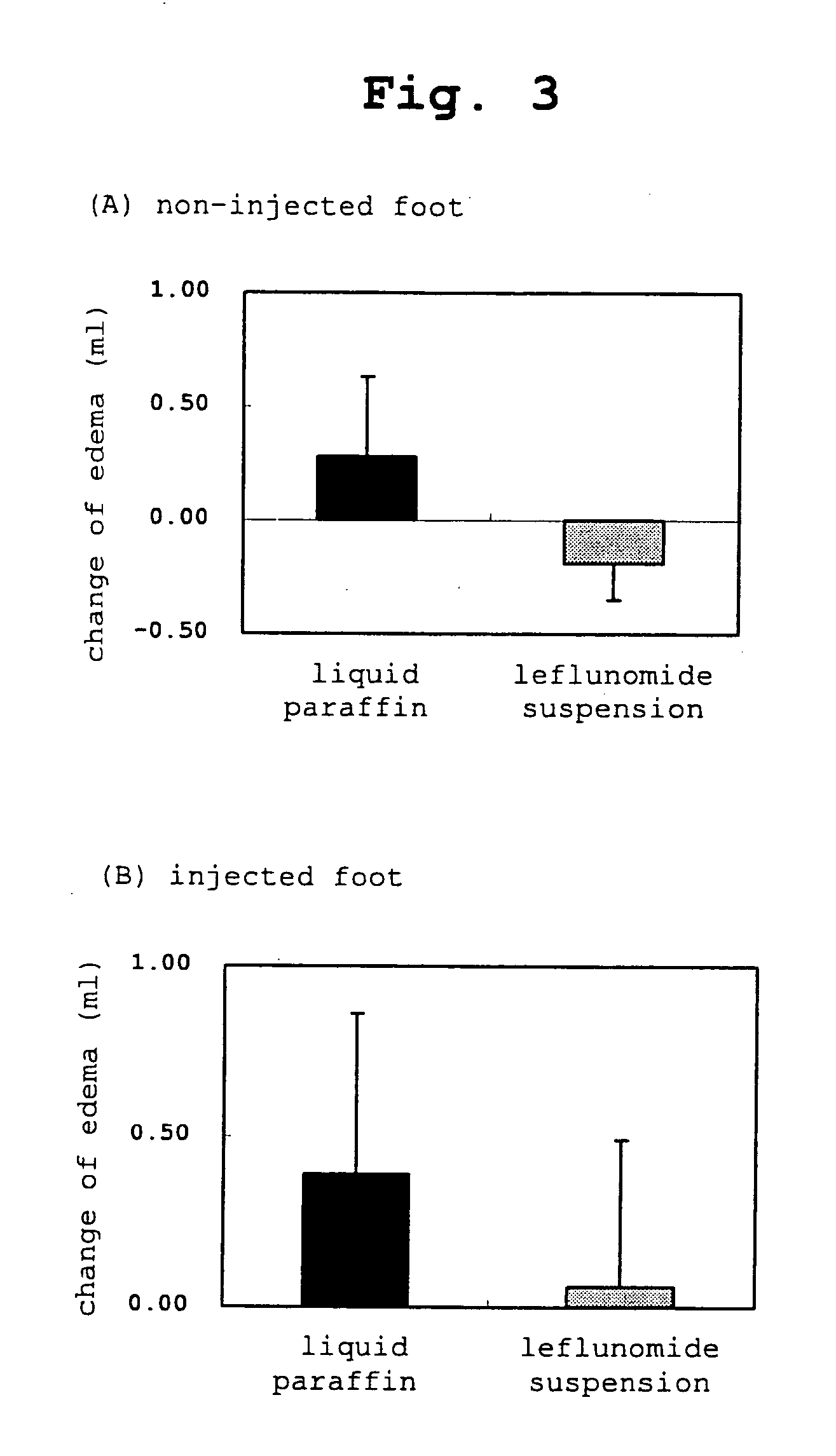
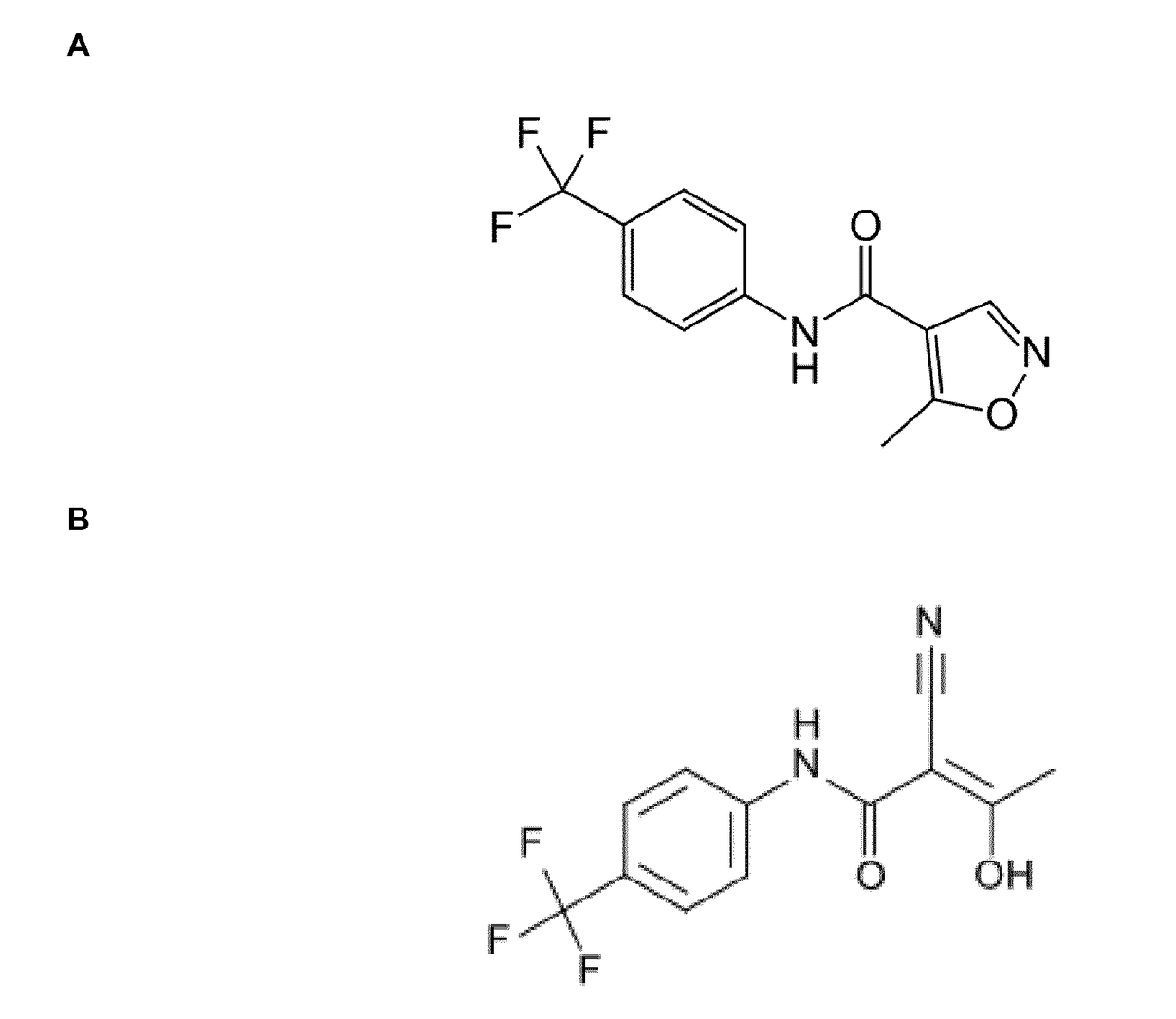
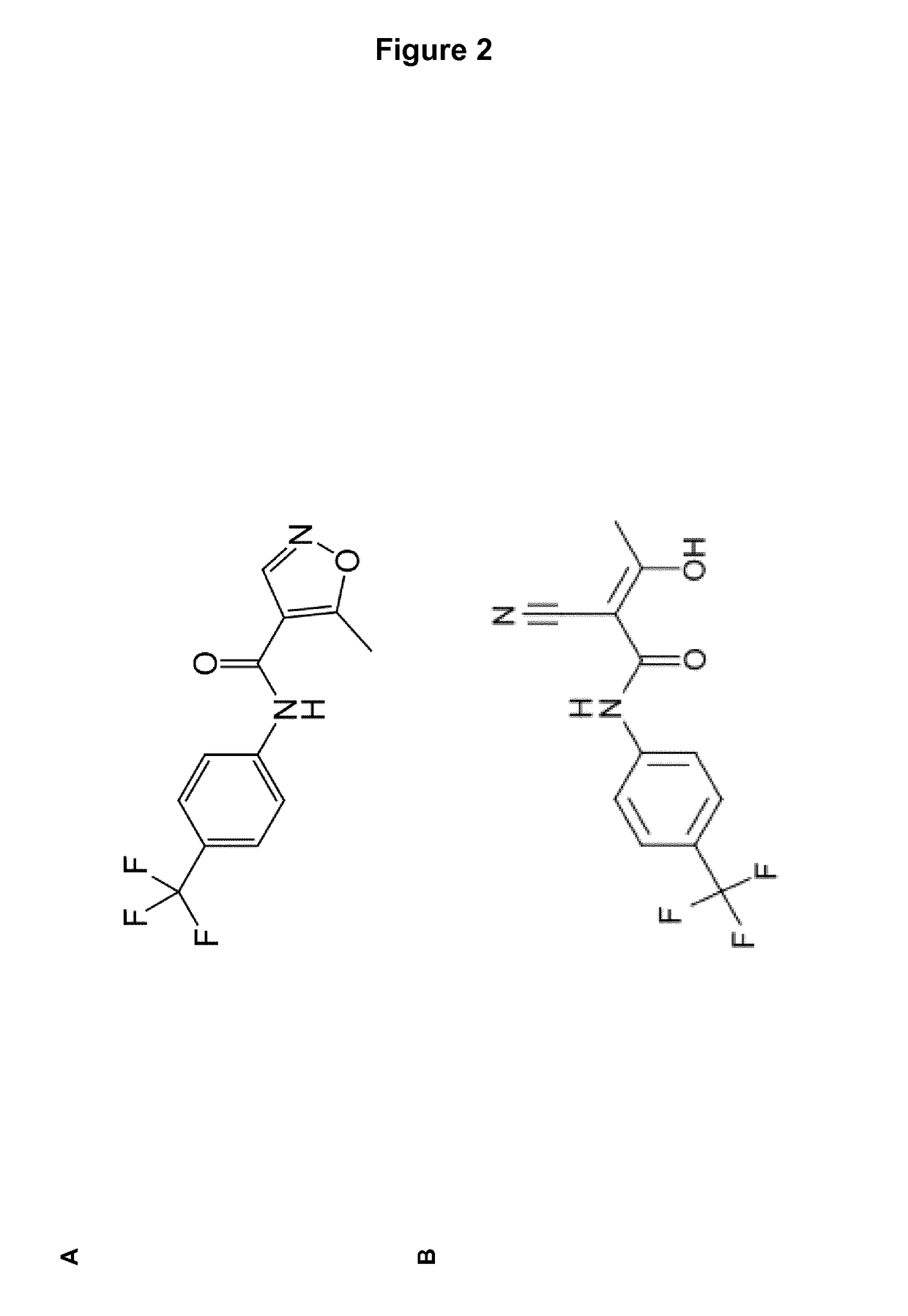
The present invention relates to a transdermal administration preparation for external application such as ointment, cream and the like, which contains SMP-114 or leflunomide or a pharmaceutically acceptable acid addition salt thereof as an active ingredient. The present invention further relates to a pharmaceutical composition for transdermal administration which contains, a) as an active ingredient, N-(4-trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide or an active motabolite thereof or a pharmaceutically acceptable salt thereof; and B)(1) a carrier for transdermal administration which contains a base for dissolution in a proportion of not less than 40 w / w %, or (2) a carrier for transdermal administration which contains a hydrophobic base for suspension having no polar group in a molecule in a proportion of not less than 70 w / w %. According to the present invention, a novel means of transdermal administration of SMP-114 or leflunomide or an active motabolite thereof or a pharmaceutically acceptable acid addition salt thereof can be provided.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Novel antiviral medicines and application thereof
PendingCN108721281ABroad-spectrum and excellent antiviral activityLow toxicityAntiviralsNitrile/isonitrile active ingredientsLeflunomideRNA virus
The invention discloses application of leflunomide, teriflunomide, brequinar and derivatives thereof to treatment of virus infection, especially RNA virus infection. RNA viruses include but are not limited to influenza viruses, respiratory syncytial viruses, hand, foot and mouth viruses (EV71), dengue viruses (type-2 dengue viruses), Zika viruses and Japanese encephalitis viruses. The medicines have broad-spectrum and excellent antiviral activity and have relatively low toxicity to normal cells.
Owner:EAST CHINA UNIV OF SCI & TECH
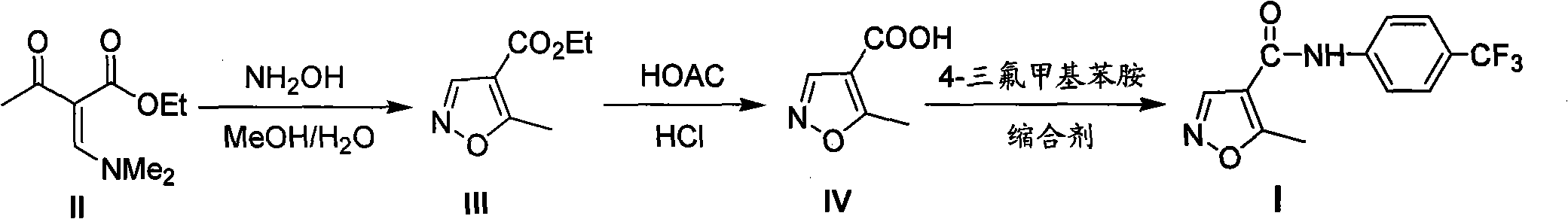
Novel environment-friendly process for preparing leflunomide
ActiveCN101817798AReduce contentHigh regional selectivityOrganic chemistryAntipyreticIndustrial effluentLeflunomide
The invention relates to a novel environment-friendly process for preparing leflunomide, which can better control the content of 3-methylisomer and 4-trifluoromethylaniline in the leflunomide, has higher yield and is simpler. The process generates little industrial effluent and exhaust gas, is more environment-friendly and can effectively reduce production cost and corrosion to equipment.
Owner:CHANGZHOU YABANG PHARMA
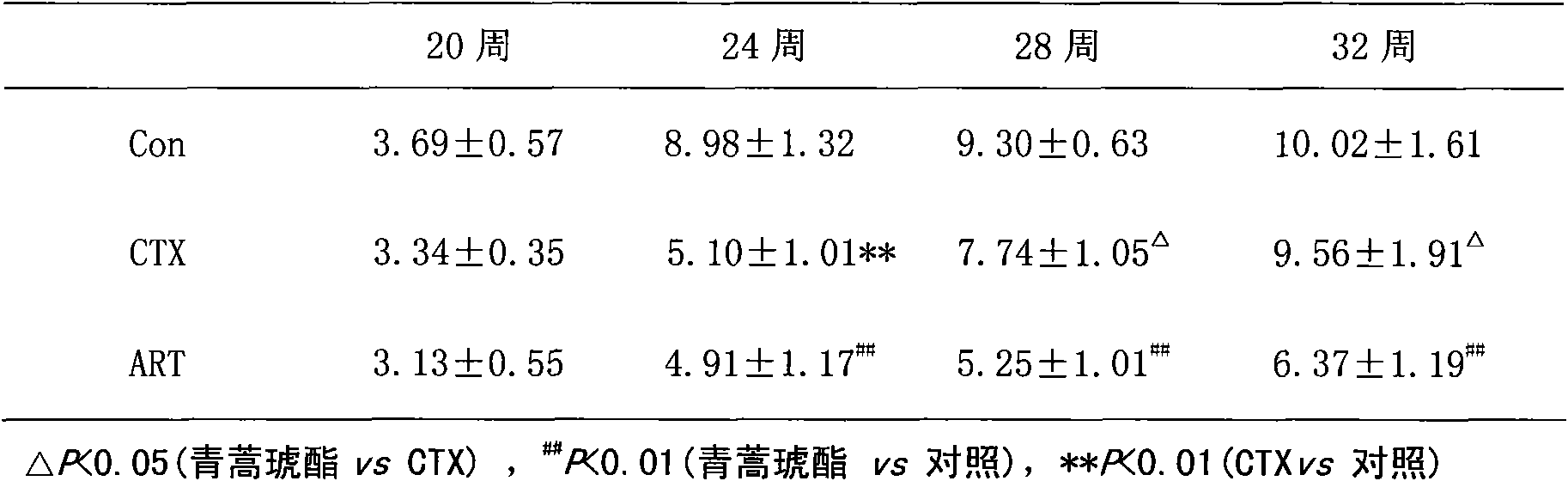
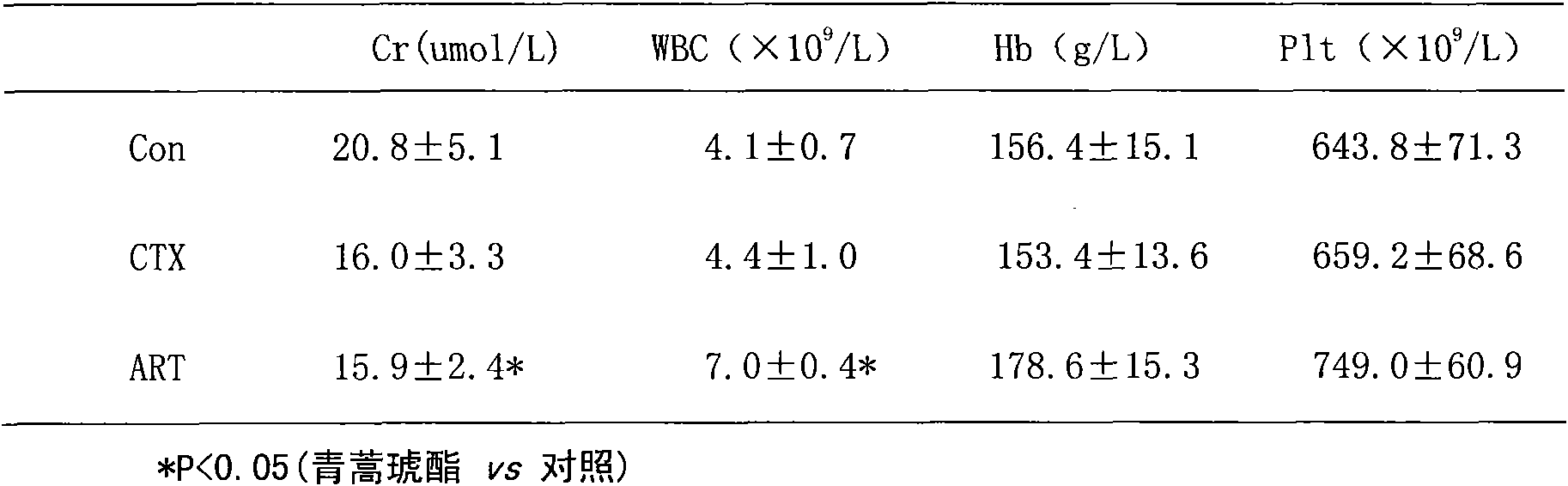
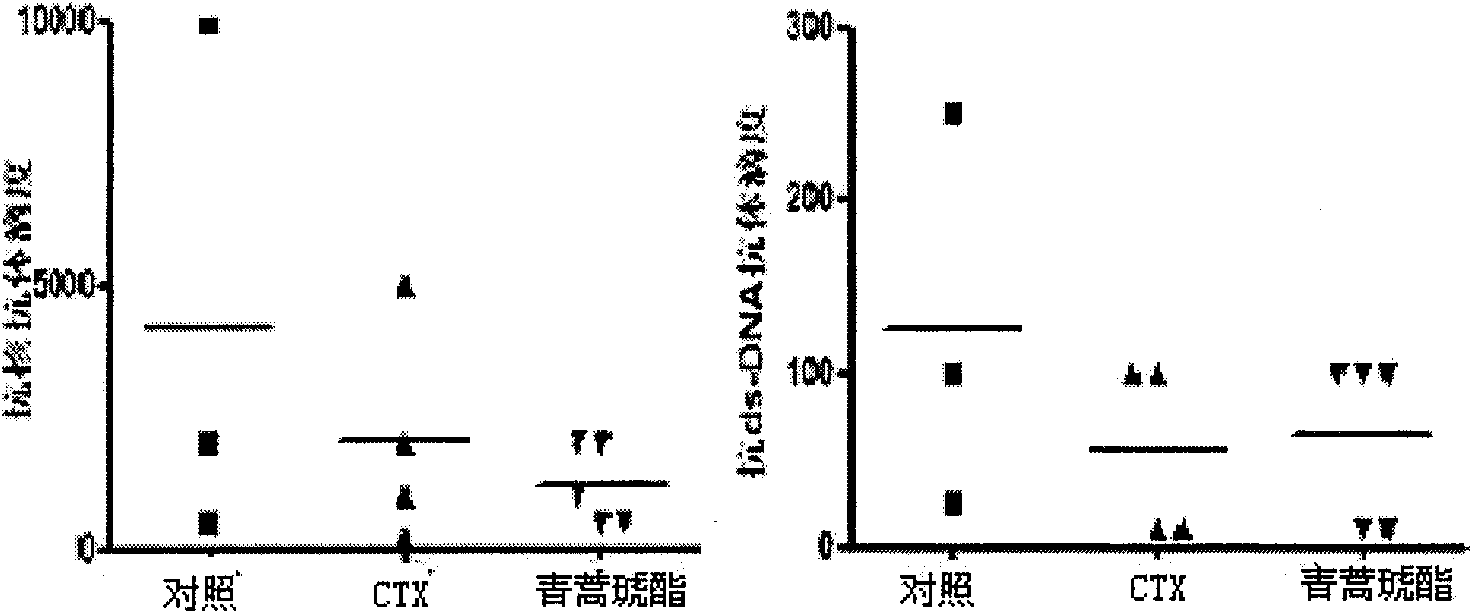
Application of artesunate as drug for treating systemic lupus erythematosus
InactiveCN101632657AMature production processQuality improvementOrganic active ingredientsSkeletal disorderLupus pernioSide effect
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
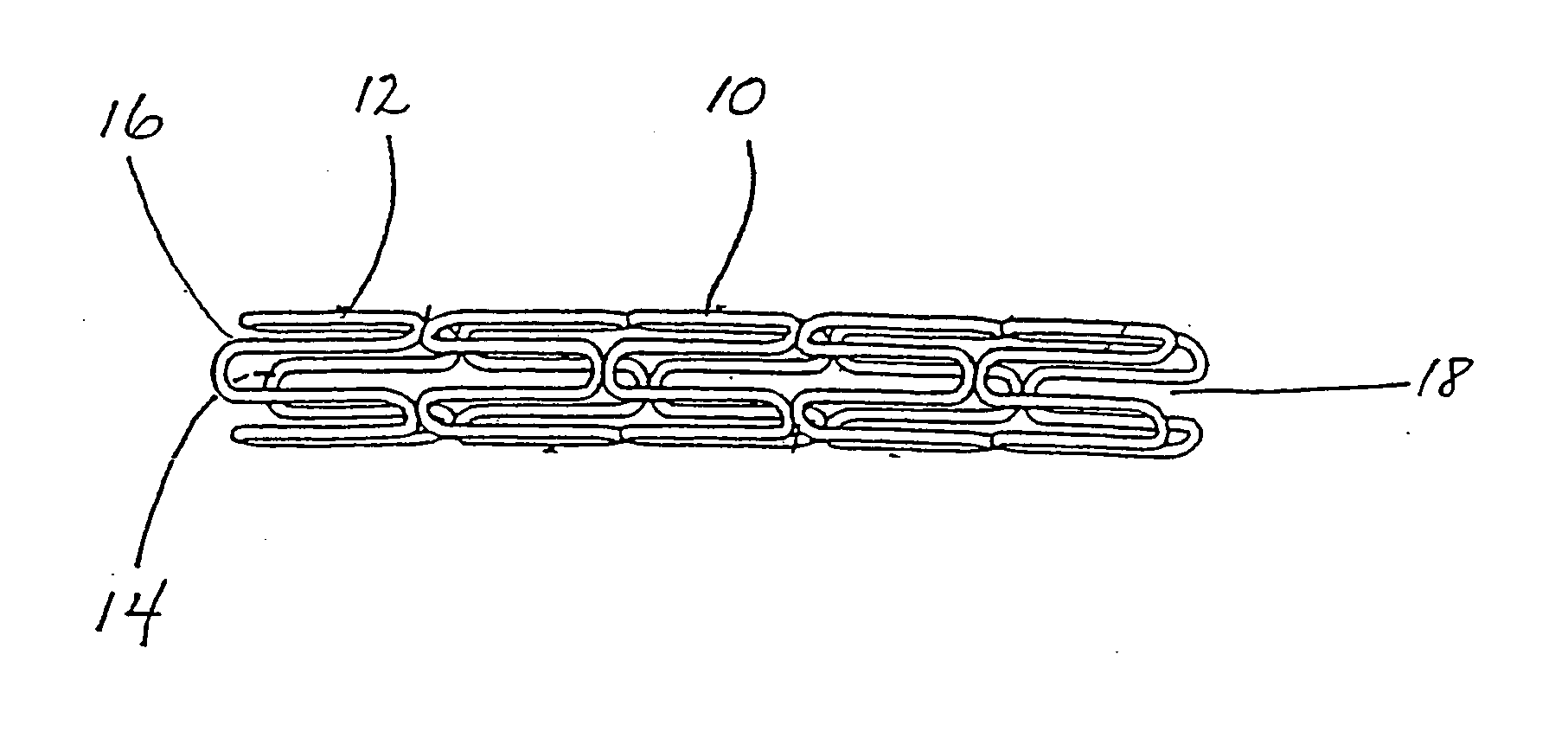
Medical devices to prevent or inhibit restenosis
InactiveUS20050261762A1Highly effective at preventing or inhibiting restenosisStentsBlood vesselsPercent Diameter StenosisLeflunomide
Implantable medical devices having anti-restenotic coatings are disclosed. Specifically, implantable medical devices having coatings of certain anti-inflammatory agents, are disclosed. The-anti-inflammatory agents are selected from the group consisting of ENMD-0997, gusperimus hydrochloride, BMS-561392, CP-461, RDP-58, CNI-1493, CEP-1347, CMT-3, prinomastat, rebimastat, leflunomide, BX-471, DF-1681, BXT-51072, M-40403, LY-293111 sodium, and pharmaceutically acceptable derivatives thereof. The anti-restenotic medical devices include stents, catheters, micro-particles, probes and vascular grafts. Intravascular stents are preferred medical devices. The medical devices can be coated using any method known in the art including compounding the anti-inflammatory agent with a biocompatible polymer prior to applying the coating. Moreover, medical devices composed entirely of biocompatible polymer-anti-inflammatory agent blends are disclosed. Medical devices having a coating comprising at least one anti-inflammatory agent in combination with at least one additional therapeutic agent are also disclosed. Furthermore, related methods of using and making the anti-restenotic implantable devices are disclosed.
Owner:MEDTRONIC VASCULAR INC
Novel Selection Marker for Cell Transfection and Protein Production
ActiveUS20170335292A1Inhibit cell proliferationInhibit DNA synthesisPeptide/protein ingredientsOxidoreductasesLeflunomideMetabolome
The present invention is within the field of industrial protein production. The invention provides a novel expression system using dihydroorotate dehydrogenase (DHODH) as a selection marker in combination with leflunomide or a metabolite thereof, notably for use in mammalian cell lines. Expression vectors encoding DHODH, cell lines comprising said vectors and methods of producing recombinant proteins are also provided.
Owner:SANOFI SA
Leflunomide tablet and preparation technology thereof
InactiveCN103989675AQuality improvementRapid dissolutionAntipyreticAnalgesicsMagnesium stearateDissolution
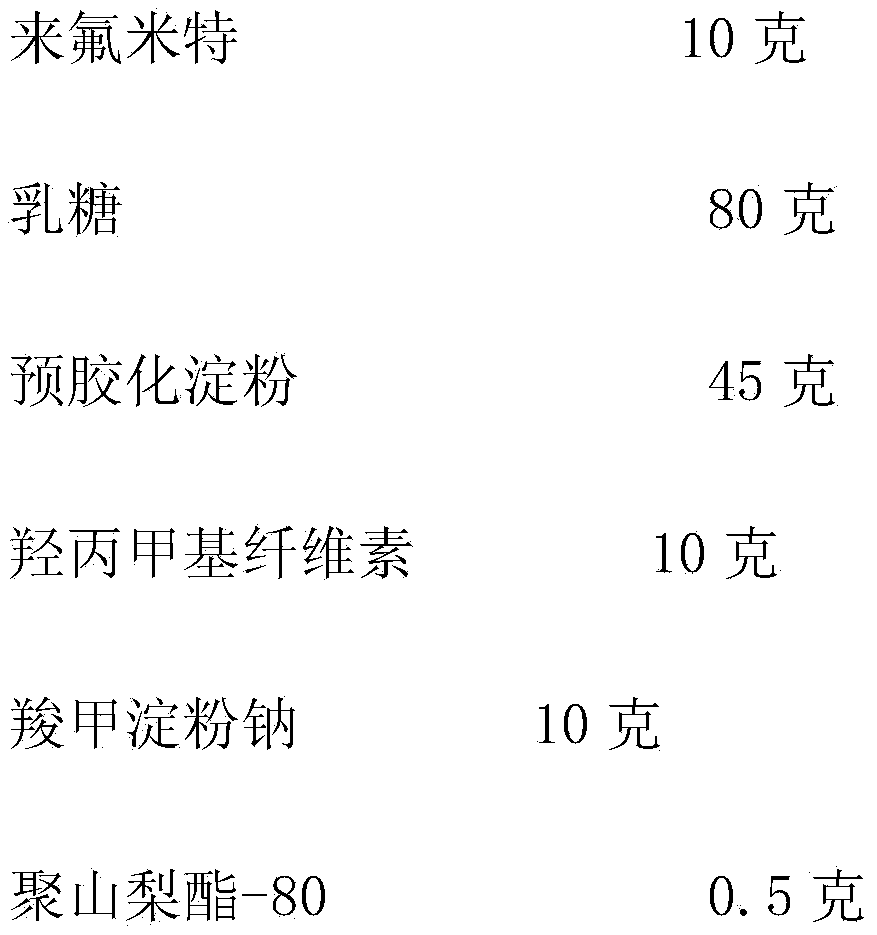
The invention discloses a leflunomide tablet and a preparation technology and use thereof. The product is prepared from leflunomide, milk sugar, pregelatinized starch, hydroxypropyl methyl cellulose, carboxymethyl starch sodium, polysorbate-80, 12% slushing pregelatinized starch, magnesium stearate and a gastric soluble film coating premix. The production method comprises the steps of fabricating tablets and coatings, and the like. The leflunomide tablet disclosed by the invention is high in dissolution rate, good in stability, good in anti-inflammatory analgesic effect, and small in effect on erythrocyte sedimentation rate, and the total effective rate is 85%. The drug disclosed by the invention has the characteristics of being fewer in medication administration times, stable in plasma concentration, long in lasting time and the like.
Owner:王俊国
Crystal form compound of leflunomide, and preparation method and application thereof
ActiveCN107311954AImprove stabilityStable in natureAntipyreticOrganic chemistry methodsLeflunomideMedicinal chemistry
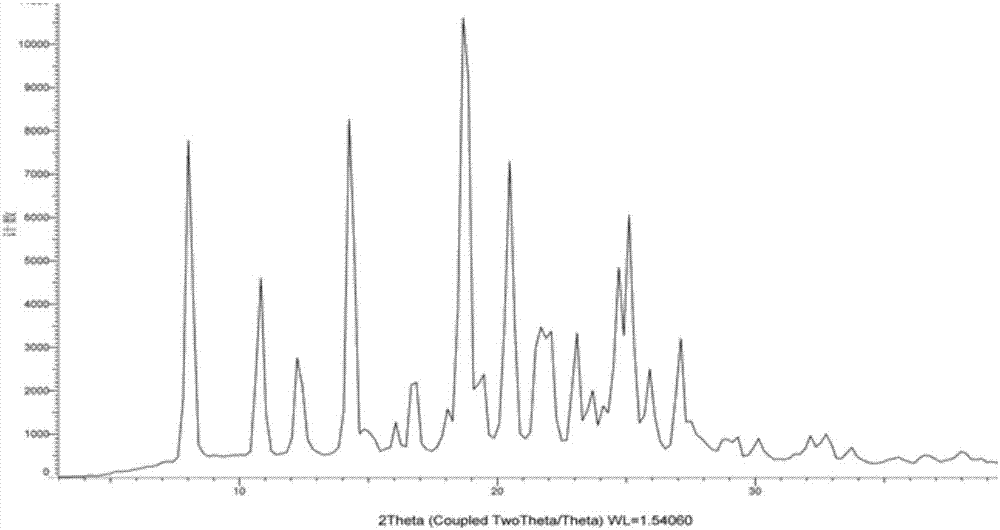
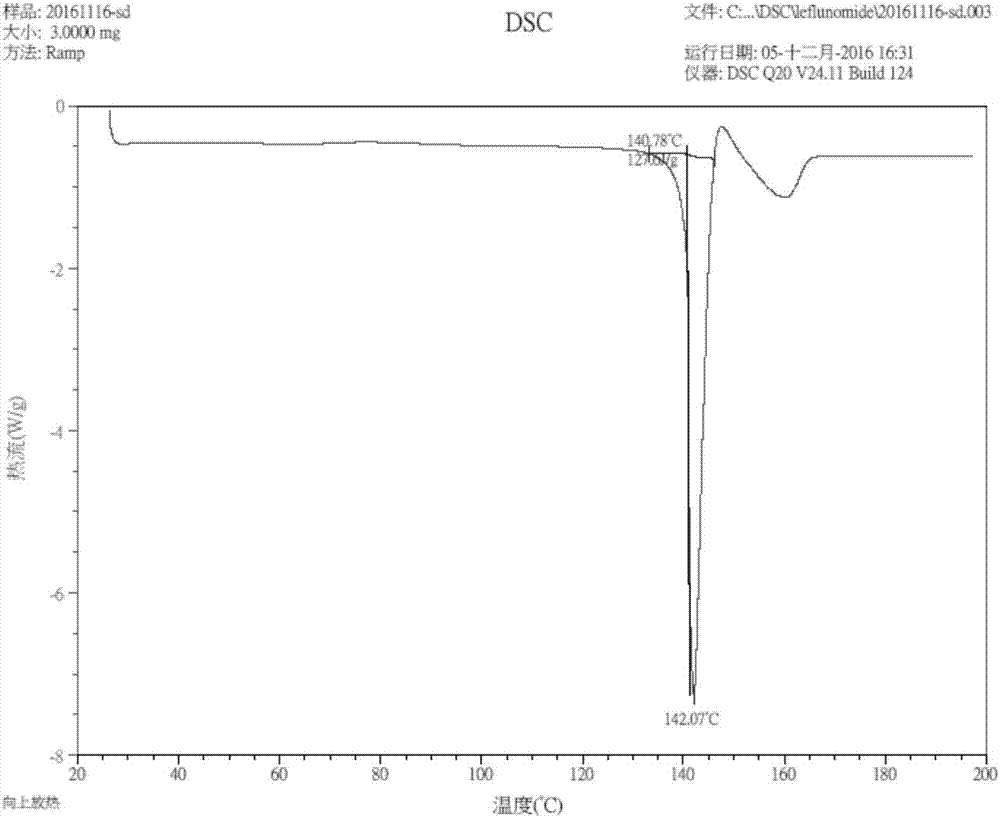
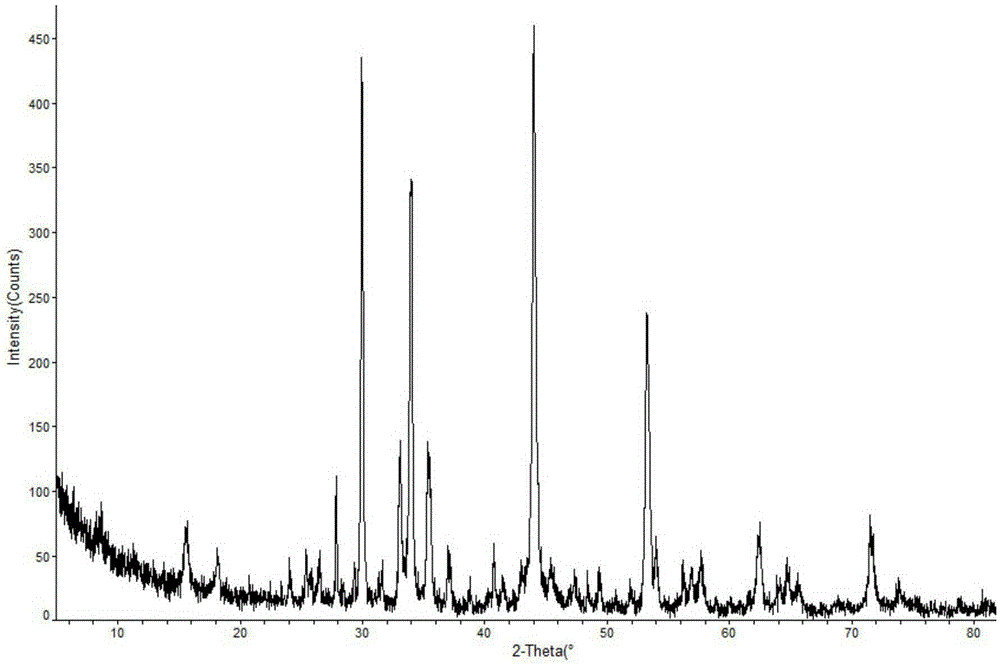
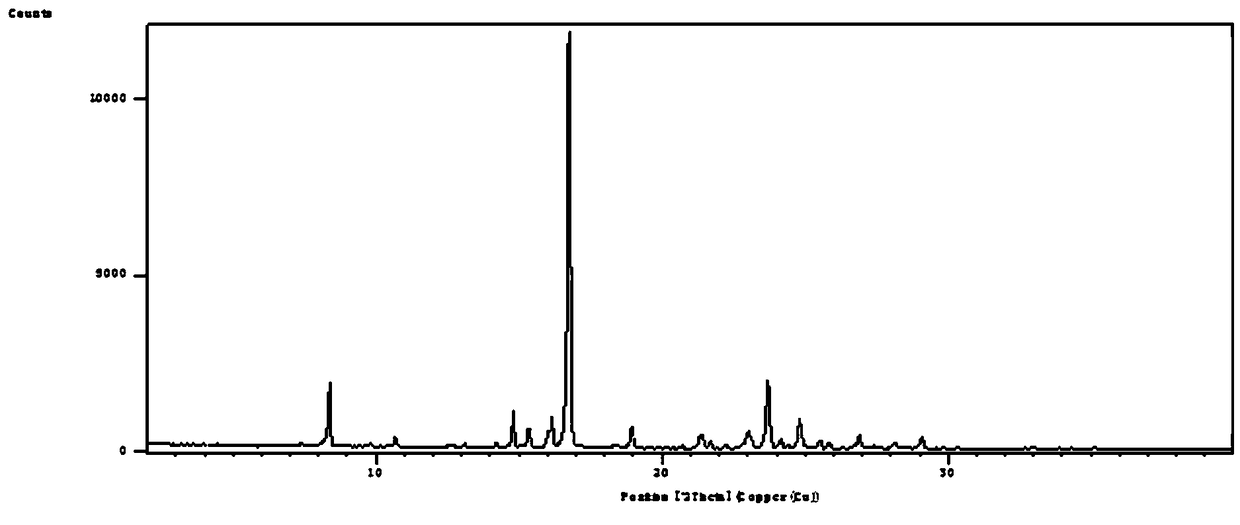
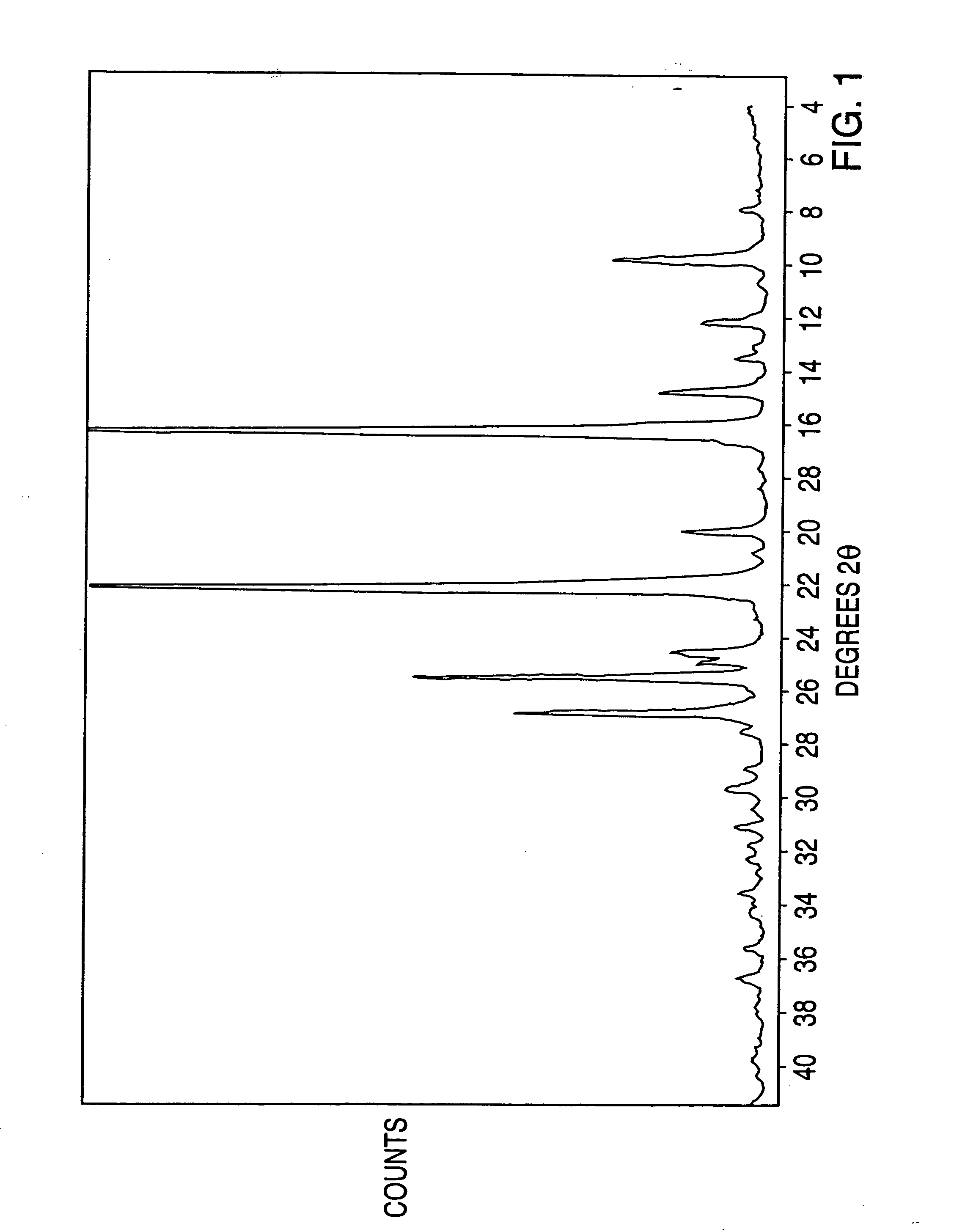
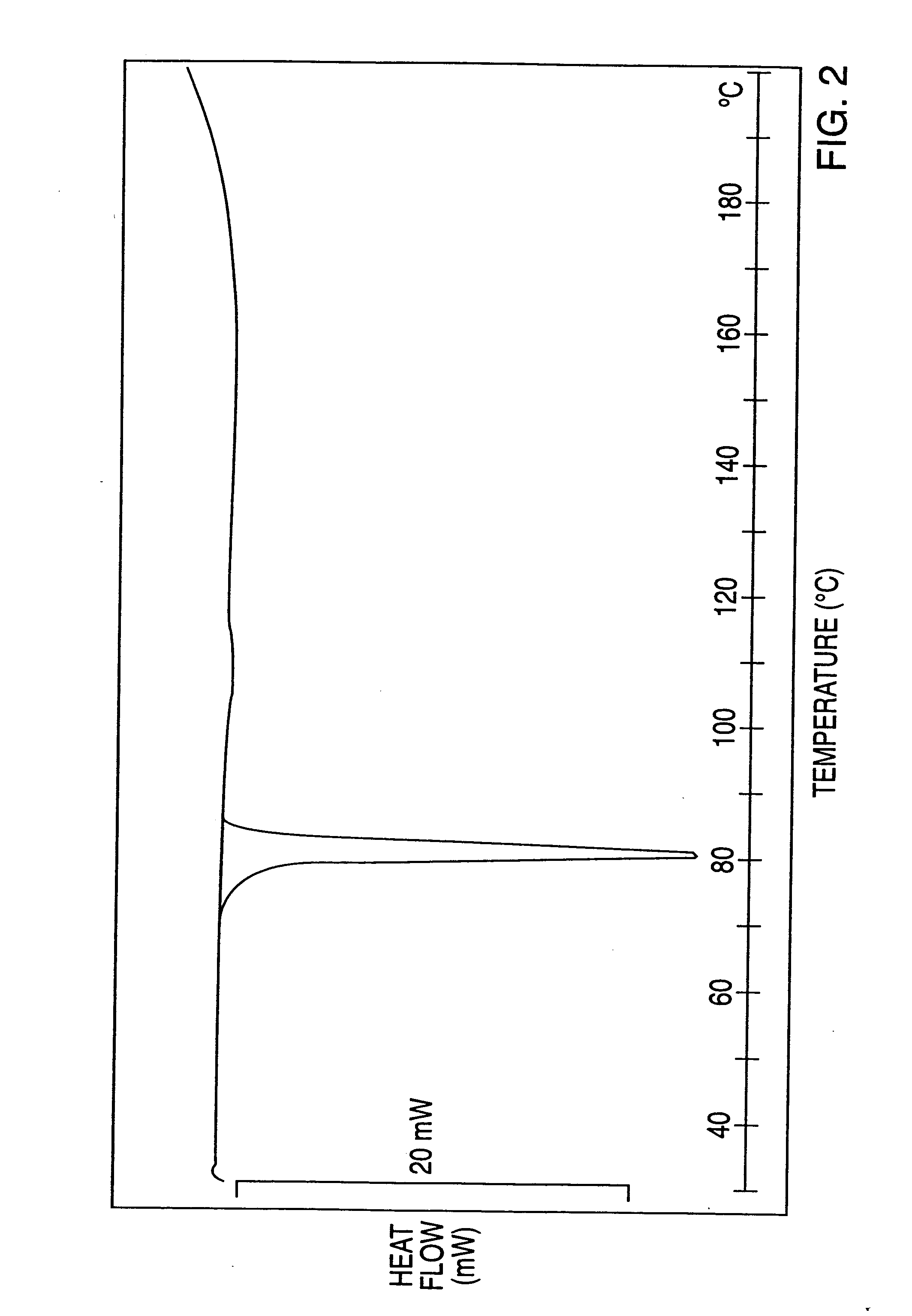
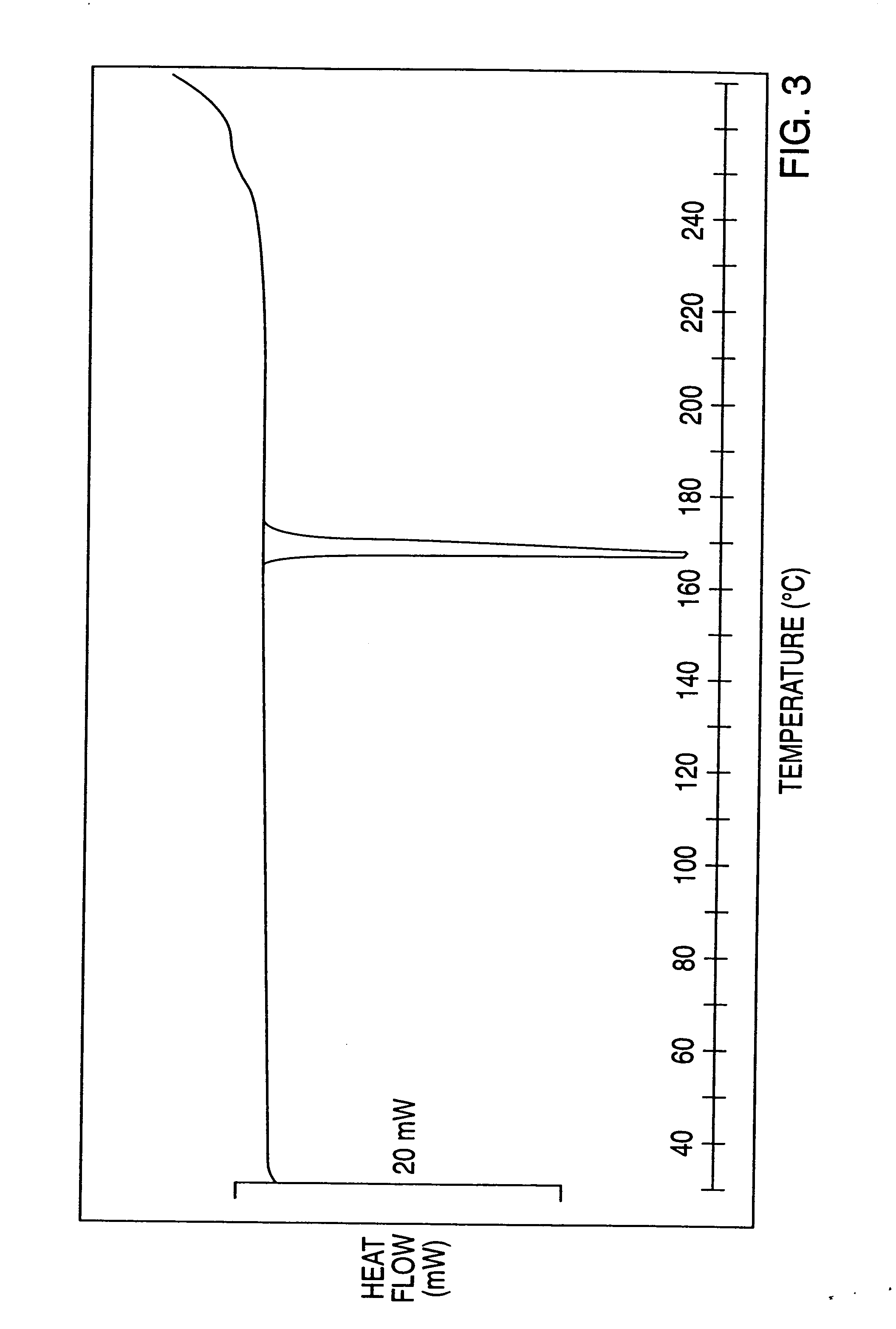
The invention discloses a crystal form compound of leflunomide. In an XRD pattern of the crystal form compound, characteristic diffraction peaks occur when the value of 2theta is 8.04 DEGs + / - 0.2 DEG, 10.83 DEGs + / - 0.2 DEG, 14.29 DEGs + / - 0.2 DEG, 18.72 DEGs + / - 0.2 DEG, 20.48 DEGs + / - 0.2 DEG and 24.92DEGs + / - 0.2 DEG. The novel crystal form of leflunomide has better stability than all the reported crystal forms of leflunomide and has high purity and good reproducibility. The invention also discloses a preparation method for the crystal form compound. The preparation method is simple in process and can be easily implemented. The invention further provides application of the crystal form compound in the fields of preparations and medicines.
Owner:力赛生物医药科技(厦门)有限公司 +1
Use of tyrosine kinase inhibitor to treat diabetes
InactiveCN1794995ACyclic peptide ingredientsHeterocyclic compound active ingredientsDiabetes mellitusTyrosine-kinase inhibitor
The present invention relates to c-Abl-, PDGF-R- or c-kit-tyrosine kinase inhibitors such as 4-(4-methylpiperazin-1-ylmethyl)-N-[4-methyl-3 -((4-pyridin-3-yl)pyrimidin-2-ylamino)phenyl]-benzamide, bis(1H-2-indolyl)-1-methanone, AG1295, CT52923, RP-1776 , GFB-111, pyrrolo[3,4-c]-β-carboline-diketones, SU 102, AG1296, RPR101511A, CDP 860, Zvegf3, CP 673451, PD 170262, KI 6783, KN 1022, AG 13736 , CHIR 258, MLN 518, SU 11248, leflunomide or their pharmaceutically acceptable salts in the preparation of medicines for treating diabetes such as type I diabetes and type II diabetes.
Owner:罗伯特・佩尔・黑格奎斯特 +1
Leflunomide sustained-release dropping pills and preparation method thereof
The invention discloses a medicine composition used for curing rheumatoid arthritis. The invention aims at making up the deficiency of the existing oral medicine (leflunomide troche) used for curing rheumatoid arthritis, and provides a leflunomide sustained-release pill that has the advantages of high bioavailability, rapid effect, high medication content, enough medicine release, controllable medicine release, few times needed by the patient to take the medicine, convenient medicine taking, low price and no pollution in the production. The leflunomide sustained-release pill related by the invention takes leflunomide as raw material and can be prepared together with medicated carrier that takes hydrophilic framework material and hydrophobic framework material as stroma.
Owner:北京博智绿洲医药科技有限公司
Molecular cloning and identifying method for silkworm gene
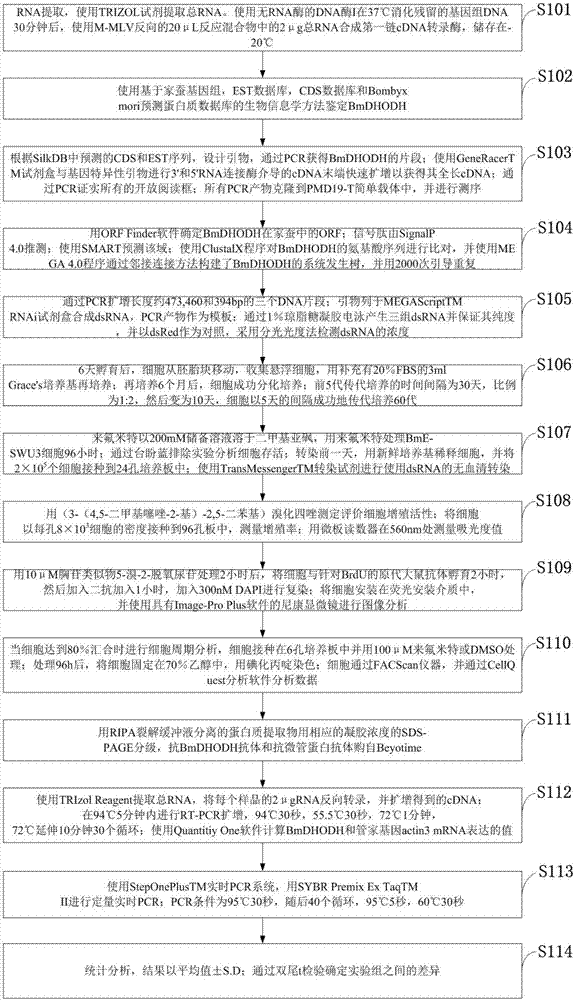
ActiveCN107384882AAffect growth and proliferationRich basic knowledgeMicrobiological testing/measurementOxidoreductasesPsocopteraTheoretical methods
The invention belongs to the field of biology and discloses a molecular cloning and identifying method for a silkworm gene. The sequence of the silkworm gene is SEQ ID NO:1; and the silkworm gene is named BmDHODH. The method disclosed by the invention has the advantages that leflunomide is used for treating silkworm cells and has great inhibitory effect on BmDHODH expression and cell proliferation; as a component in pyrimidine synthesis, BmDHODH is a necessary biological enzyme closely related with cell growth and proliferation, can become a new gene target for killing lepidoptera pests, provides new opinion for eco-friendly pest control and provides a new target and a new theoretical method for developing an environment-friendly pesticide; and as a biological model for the lepidoptera pests, basic knowledge of the lepidoptera pests can be enriched for silkworm BmDHODH, and a new method is provided for environment-friendly pest control.
Owner:SOUTHWEST UNIVERSITY
Leflunomide dripping pill and its prepn
The present invention discloses one kind of medicine composition for treating rheumatoid arthritis, and aims at providing one kind of leflunomide dripping pill for treating rheumatoid arthritis with high bioavailability, fast medicine release, fast acting, high medicine content, easy taking, low cost and production process without pollution. The leflunomide dripping pill is prepared with leflunomide as medicine material and medicine carrier as matrix.
Owner:北京博智绿洲医药科技有限公司
Novel environment-friendly process for preparing leflunomide
ActiveCN101817798BReduce dosageControl contentOrganic chemistryAntipyreticIndustrial effluentLeflunomide
The invention relates to a novel environment-friendly process for preparing leflunomide, which can better control the content of 3-methylisomer and 4-trifluoromethylaniline in the leflunomide, has higher yield and is simpler. The process generates little industrial effluent and exhaust gas, is more environment-friendly and can effectively reduce production cost and corrosion to equipment.
Owner:CHANGZHOU YABANG PHARMA
High bioavailability Leflunomide tablet and preparing method thereof
InactiveCN106924713AHigh dissolution rateImprove bioavailabilityAntipyreticAnalgesicsSide effectBeta-Carotene
The invention discloses a high bioavailability Leflunomide tablet and a preparing method thereof. The Leflunomide tablet is prepared from, by weight, 1-5 parts of Leflunomide, 5-60 parts of filler, 1-15 parts of disintegrating agent, 0.5-5 parts of lubricant, 0.1-2 parts of surface active agent, 2-10 parts of adhesive, 0.1-5 parts of threonine, 1-3 parts of glutamine, 1-5 parts of beta-carotene and 0.1-2 parts of glutathione. The Leflunomide tablet is high in dissolution rate and bioavailability and little in side effect, and has a positive meaning of ensuring safety and effectiveness of clinical medication.
Owner:FUJIAN HUITIAN BIOLOGICAL PHARMA
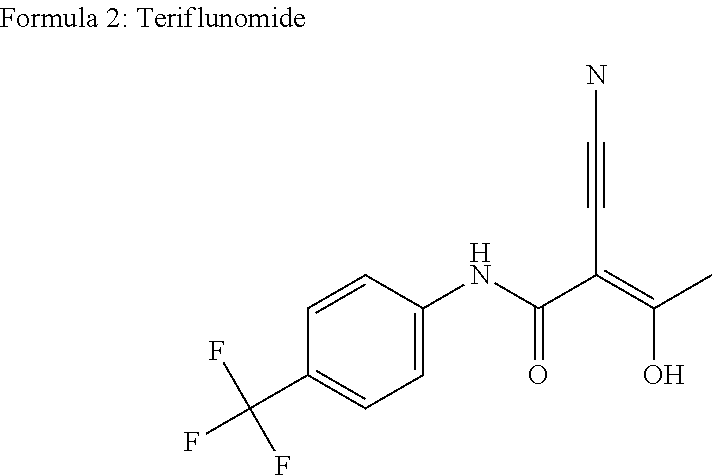
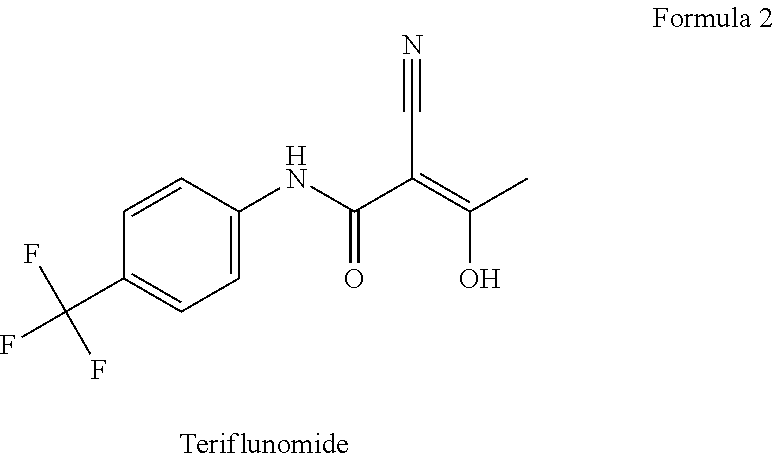
Application of sodium teriflunomide to preparation of medicine for treating autoimmune diseases
InactiveCN105395539AFast absorptionImprove bioavailabilityNervous disorderAntipyreticActive componentAutoimmune disease
The invention discloses an application of sodium teriflunomide, which is a sodium salt with low hepatotoxicity of a leflunomide active metabolite, to preparation of a medicine for treating autoimmune diseases. Compared with leflunomide or teriflunomide, sodium teriflunomide has lower hepatotoxicity and a larger therapeutic window. Sodium teriflunomide or a medical combination taking sodium teriflunomide as an active component is used as an immunosuppressor, and is used for treating autoimmune diseases.
Owner:CINKATE PHARMA INTERMEDIATES
Method for the preparation of teriflunomide
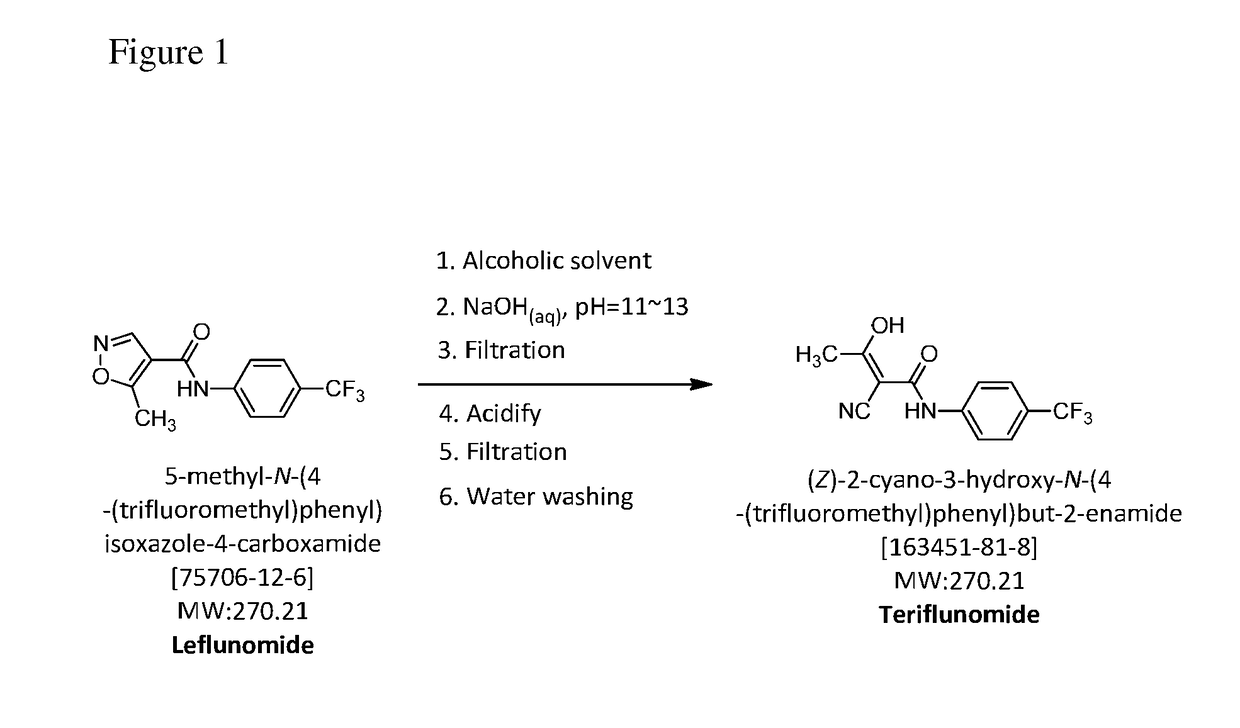
InactiveUS20170073304A1Feasible at commercial scaleCarboxylic acid nitrile preparationOrganic compound preparationAlcoholAqueous sodium hydroxide
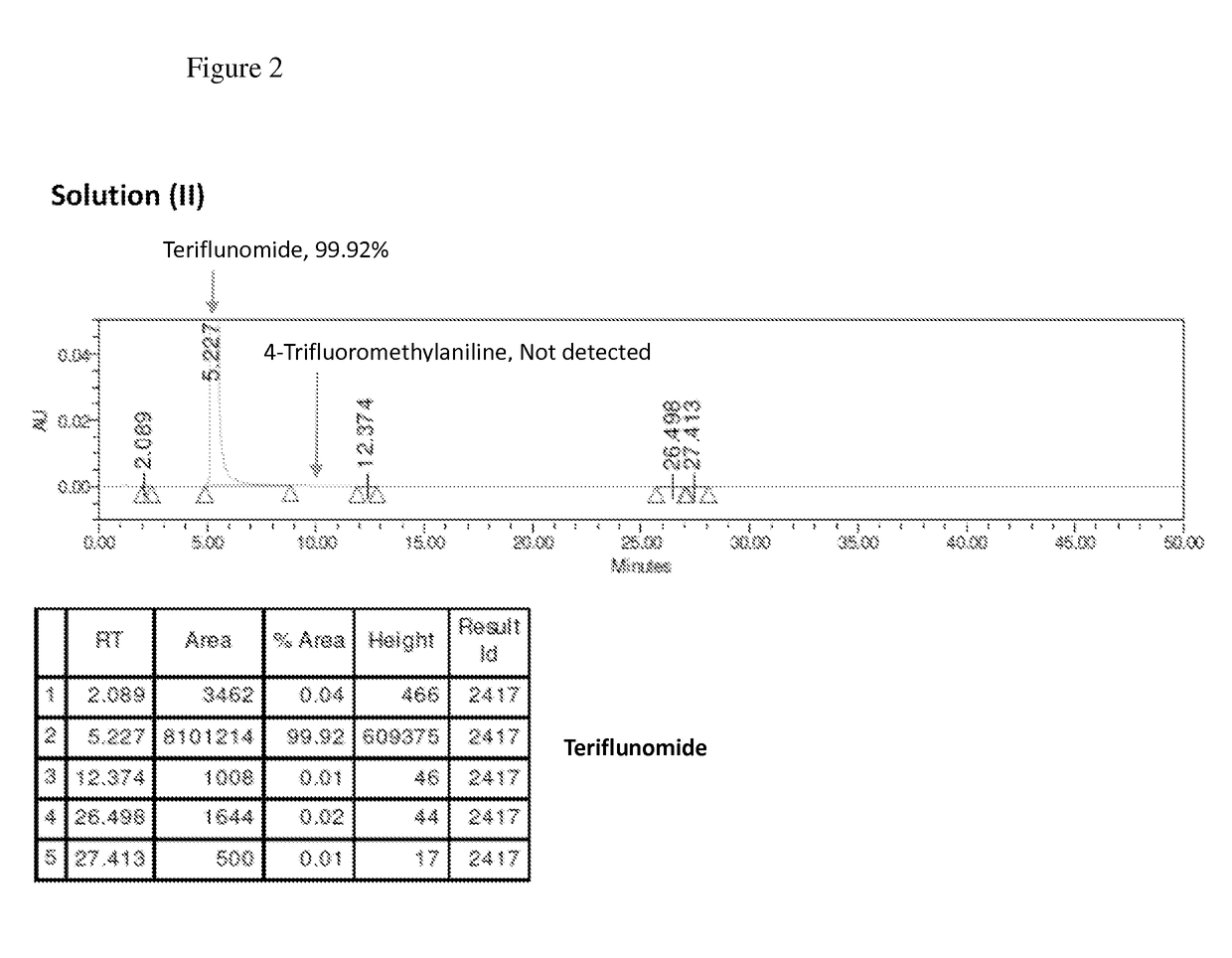
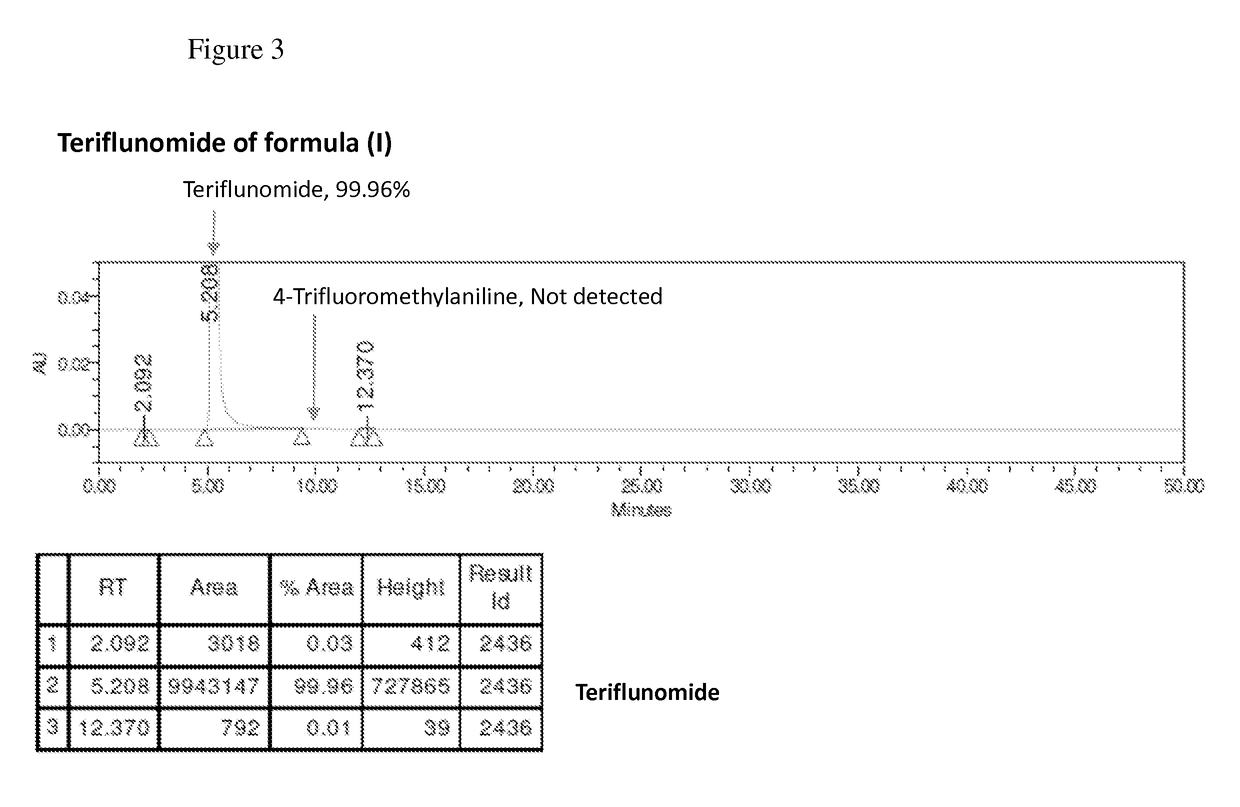
The present invention relates to a method for preparation of Teriflunomide, comprising steps of: (a) adding Leflunomide to an alcoholic solvent to give solution (I); (b) adding an aqueous sodium hydroxide solution slowly into the solution (I) to give solution (II); (c) acidifying the solution (II) with inorganic acid for precipitation to give solution (III); and (d) filtering the solution (III) to give Teriflunomide.
Owner:FORMOSA LAB
Leflunomide tablet preparation and preparation method thereof
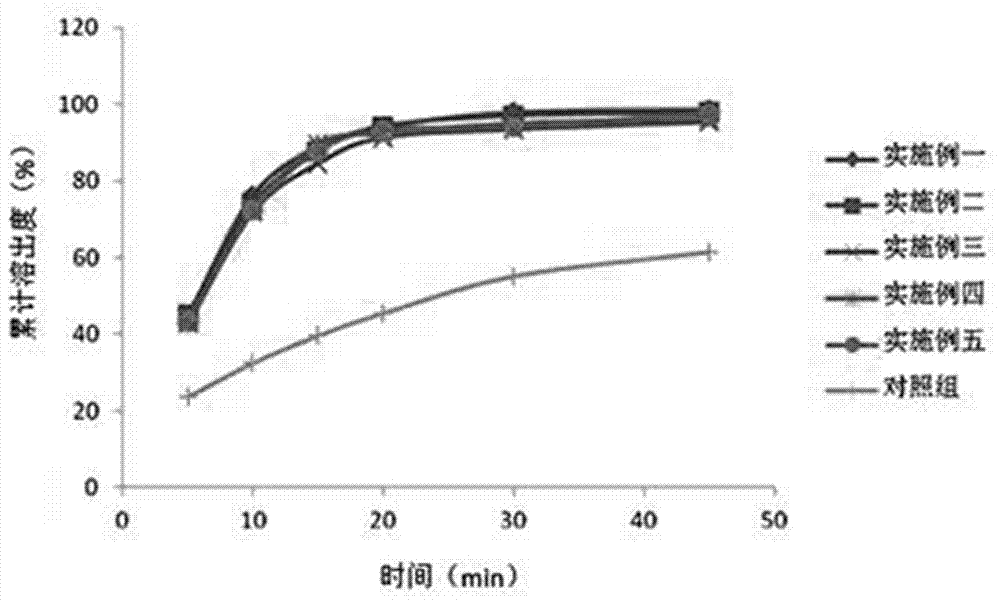
The invention relates to a leflunomide tablet preparation and a preparation method thereof. The solid dispersion method is adopted for improving the dissolution rate of a drug, thereby improving the bioavailability of the drug. The in vitro dissolution experiment shows that the dissolution rate of leflunomide tablets prepared by the method can be more than 90% at 45min under the condition of taking water as a dissolution medium.
Owner:JIANGSU YABANG AIPUSEN PHARMA
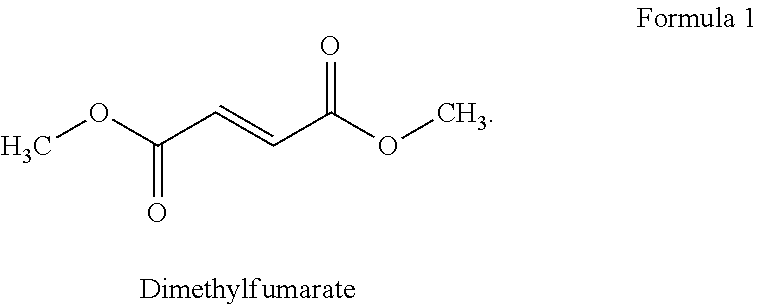
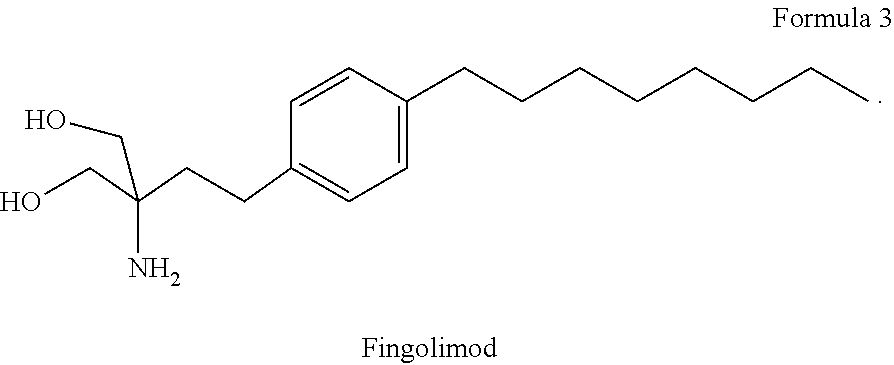
Combination therapy for treatment of multiple sclerosis
InactiveUS20150164849A1Good effectIncrease in severe adverse eventBiocideNervous disorderSide effectTolerability
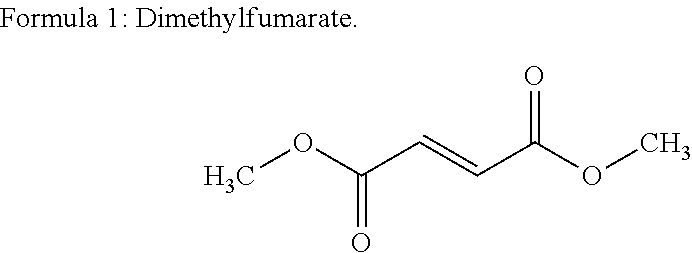
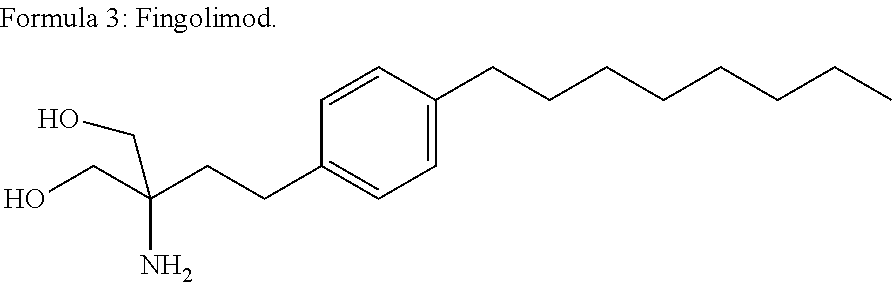
The present invention relates to a method of treating MS in a human patient in need of such treatment and comprises administering to said patient a combination therapy in a single oral dosage form (e.g. a tablet or capsule) of dim ethyifurrta rate and one agent selected from teriflunomide (or its prodrug leflunomide), fingolimod and laquinimod. This combination is more effective than the single agents alone and / or has reduced side effects and better tolerability than the single agents alone and / or can be given in a reduced frequency. Moreover, the present invention is directed to a pharmaceutical composition suitable for the oral treatment of multiple sclerosis consisting of dimethylfumarate and one agent selected from teriflunomide, fingolimod and laquinimod as active ingredients and one or more pharmaceutically acceptable excipients.
Owner:BIOGEN SWISS MFG GMBH
Leflunomide tablet for treating adult rheumatoid arthritis
InactiveCN105287420AImprove stabilityHigh dissolution rateOrganic chemistryAntipyreticPolyethylene glycolLeflunomide
The invention discloses a leflunomide tablet for treating adult rheumatoid arthritis, and belongs to the technical field of medicines. The composition is prepared from leflunomide, lactose, starch, hydroxy propyl cellulose, lauryl sodium sulfate, superfine silica powder, polyethylene glycol 6,000, sodium carboxymethyl starch and magnesium stearate. The leflunomide is a novel crystal compound. An experiment shows that the medicine is fast to dissolve, simple in preparation technology and applicable to mass production.
Owner:李正梅
Prophylactic and/or therapeutic method for rheumatoid arthritis
InactiveUS20090281106A1Enhanced inhibitory effectLess side effectsBiocideAntipyreticSide effectMedicine
The present invention provides a prophylactic and / or therapeutic agent for rheumatoid arthritis comprising 2-benzyl-5-(4-chlorophenyl)-6-[4-(methylthio)phenyl]-2H-pyridazin-3-one and leflunomide in combination.The prophylactic and / or therapeutic agent of the present invention can be administered orally, exhibits an excellent arthritis suppressive effect with fewer side effects, and is therefore useful for the prophylaxis and / or treatment of rheumatoid arthritis.
Owner:KOWA CO LTD
Application of leflunomide and teriflunomide to leukemia treatment
InactiveCN108721280AInhibitory activityAntineoplastic agentsNitrile/isonitrile active ingredientsChronic lymphocytic leukemiaChronic granulocytic leukemia
The invention discloses application of leflunomide and teriflunomide or derivatives or pharmaceutically acceptable salt thereof to preparation of medicines for treating leukemia. The leflunomide and the teriflunomid have remarkable inhibition effect on the leukemia comprising acute lymphoblastic leukemia (ALL), acute myelocytic leukemia (AML), chronic granulocytic leukemia, chronic lymphocytic leukemia and the like; and new material basis is laid for the development of the medicines for treating leukemia.
Owner:EAST CHINA UNIV OF SCI & TECH
Application of Lavermit in preparing medicine for tetanic rachitis
The present invention provides an application of lyfluomite in preparation of medicine for curing ankylosing spondylitis. The lyfluomite can utilize its action of inhibiting the hyperlasia of fibrolast to reduce hyperplasia of fibrous tissue between joint and bone trabecula. Besides, it also can inhibit the hyperplasia of activated lymphocytes and conversion of B-lymphocyte into plasmocyte, and can reduce infiltration of lymphocyte and plasmocyte in articular synovium of ankylosing spondylitis. The therapeutic effect is reliable, and its toxic side effect is less, so it is an effective medicine for curing ankylosing spondylitis.
Owner:CINKATE PHARMA INTERMEDIATES
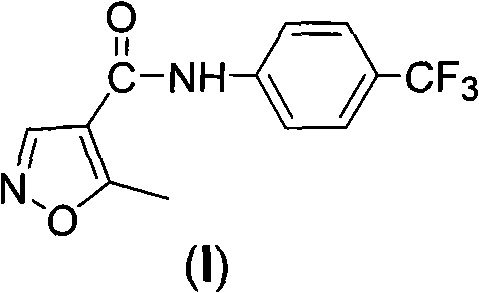
A novel process for the preparation of teriflunomide
ActiveUS20180170859A1High purityHigh yieldMaterial analysis using wave/particle radiationOrganic compound preparationCarbonyl chlorideCarboxylic acid
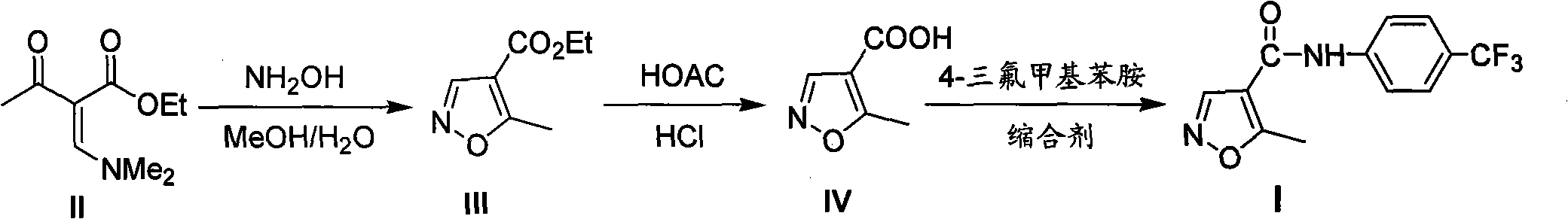
The present invention provides a process for the preparation of Teriflunomide (Formula-I). The present invention describes the synthesis of Teriflunomide without isolating the intermediate Leflunomide. Teriflunomide is prepared from 5-Methyl isoxazole-4-carboxylic acid by converting to its acid chloride and coupling with 4-trifluoromethyl aniline to obtain Leflunomide (which is not isolated) followed by ring opening reaction using aq. Sodium Hydroxide to form Teriflunomide. In other words, the process is telescoped from 5-methylisoxazole-4-carbonyl chloride.
Owner:BIOCON LTD
Drug for reducing side effects of leflunomide
InactiveCN107158107AReduce the side effects of LeformideAntipyreticAnalgesicsSide effectClinical trial
The invention discloses a drug for reducing side effects of leflunomide and belongs to the technical field of drugs. The drug for reducing the side effects of the leflunomide is prepared from leflunomide and auxiliary materials of a biologic preparation. A clinical test proves that the problem that the leflunomide easily generates the side effect during clinical treatment of polymyositis can be effectively solved.
Owner:张凤霞
Medicine for curing rheumatism and preparation method thereof
InactiveCN106377677AWide variety of sourcesSimple preparation processSalicyclic acid active ingredientsAntipyreticSide effectRheumatism
The invention discloses a medicine for curing rheumatism. The medicine is prepared form the following raw materials in parts by weight: 2 to 5 parts of an auxiliary material, 3 to 7 parts of dendrobium nobile stem solution, 1 to 2 parts of sodium salicylate, 0.2 to 1.5 parts of leflunomide, 2 to 4 parts of oryzanol, 0.2 to 1 part of vitamin E, 2 to 4 parts of lysine, 1 to 4 parts of caffeine, 4 to 9 parts of cucurbitacine, 0.5 to 2.5 parts of aspirin, 0.4 to 2 parts of terramycin, 3 to 6 parts of glycerinum and 10 to 18 parts of normal saline. The invention further discloses a preparation method of the medicine. The medicine is wide in raw material source, simple in preparation technology and suitable for large-scale industrial production; as the ingredients of the dendrobium nobile stem solution, the cucurbitacine and the sodium salicylate have an synergistic effect, the medicine is reasonable in formula, convenient to use, short in curing time, capable of curing the rheumatism completely, low in relapse possibility, free of toxic and side effects and good in use effect.
Owner:ZHENGZHOU ZHENGXIAN PHARMA CO LTD
Simple preparation method of teriflunomide
InactiveCN113072464AHigh yieldEasy to recycleCarboxylic acid nitrile preparationOrganic compound preparationLeflunomideCombinatorial chemistry
The invention provides a simple preparation method of teriflunomide, and belongs to the field of medicinal chemistry. The preparation method comprises the following steps of: (1) mixing 5-methylisoxazole-4-formic acid and a condensing agent in a solvent under an alkaline condition, and carrying out condensation reaction to obtain an active ester system; (2) mixing the active ester system and 4-trifluoromethylaniline in a solvent, and carrying out condensation reaction to obtain an intermediate leflunomide; and (3) carrying out alkali treatment and acid treatment on the obtained intermediate leflunomide to obtain teriflunomide. According to the method, the 5-methylisoxazole-4-formic acid reacts with the 4-trifluoromethylaniline in the form of active ester, so that the reaction activity of the 5-methylisoxazole-4-formic acid and the 4-trifluoromethylaniline is improved, the reaction condition is mild, the obtained intermediate leflunomide does not need to be purified, and the yield of teriflunomide is improved.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
Environmental-protection simple preparation method of teriflunomide
ActiveCN105272882AReduce usageShort reaction stepsCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventLeflunomide
The invention discloses an environmental-protection simple preparation method of teriflunomide; leflunomide is used as a starting material, a heterogeneous reaction is adopted, an organic solvent is avoided from being used in a synthesis reaction process, and thus the high-purity teriflunomide can be prepared.
Owner:CINKATE PHARMA INTERMEDIATES
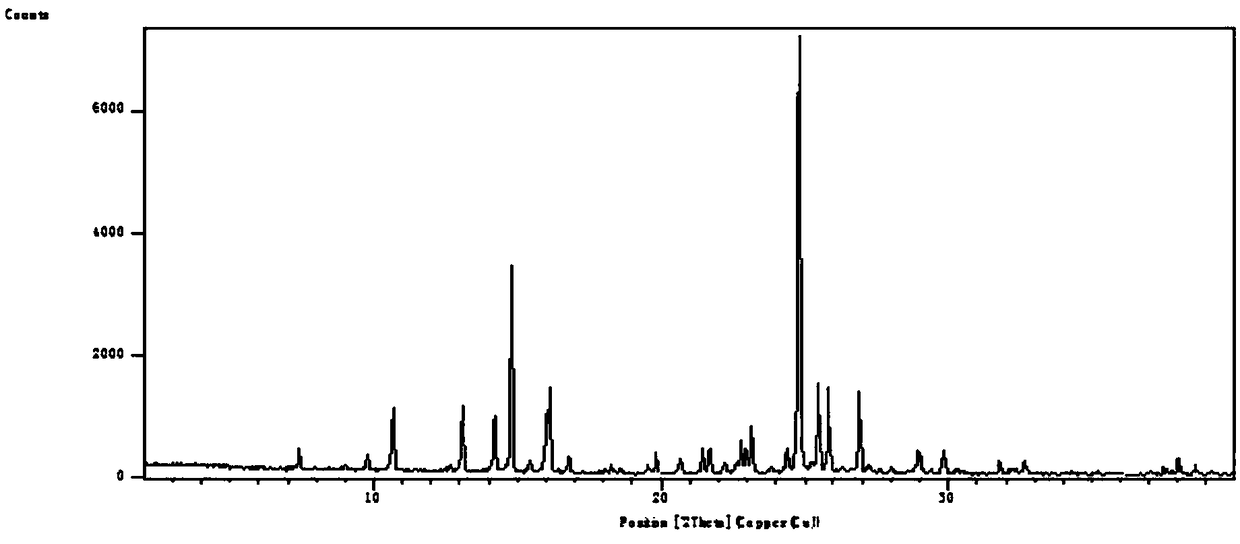
Novel crystal form compound of leflunomide and preparation method of novel crystal form compound
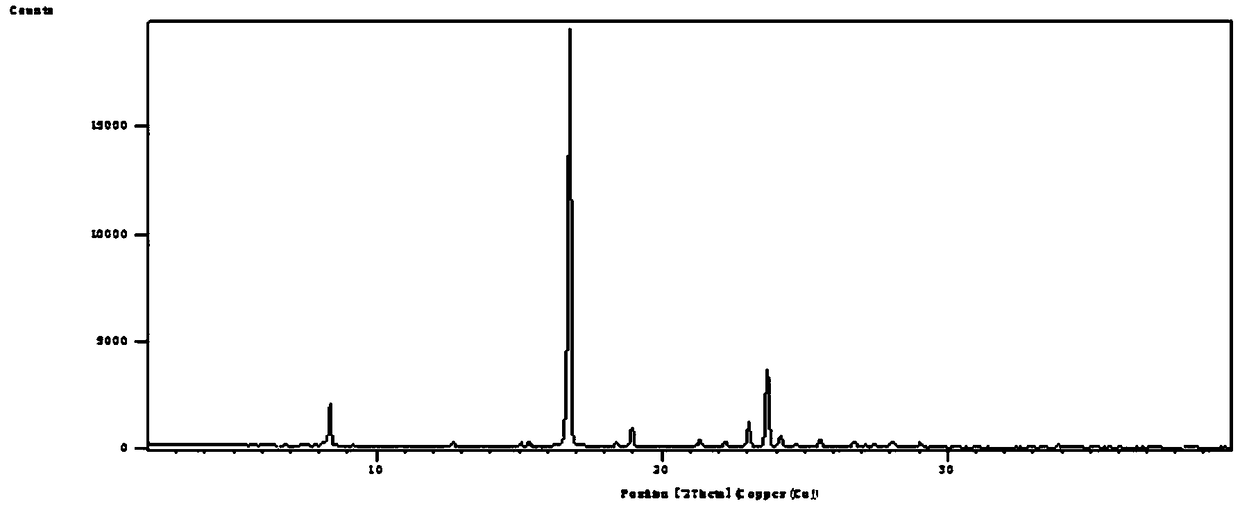
The invention discloses a crystal form compound of leflunomide V. The crystal form is different from X-ray powder diffraction and differential scanning patterns of the leflunomide, which are publishedin the existing document, and is a novel crystal form; and the X-ray powder diffraction pattern has characteristic diffraction peaks different from a reported crystal form at 2theta of 7.8255 degrees, 8.7099 degrees, 9.1789 degrees, 9.6017 degrees, 11.1488 degrees, 14.9436 degrees, 15.6434 degrees, 18.6293 degrees, 19.3055 degrees, 20.2744 degrees, 23.3166 degrees, 23.5235 degrees, 30.1244 degrees, 30.6877 degrees, 31.5469 degrees and 37.2453 degrees. The solubility of the crystal form is better than that of other all crystal forms in existing reports, the purity is high and the repeatabilityis good; and the invention further discloses a preparation method of the crystal form.
Owner:SHENZHEN NYCRIST TECH CO LTD
Combination therapy for treatment of multiple sclerosis
InactiveUS20190091190A1Good effectShow an Increase in severe adverse eventsNervous disorderAntipyreticSide effectOral treatment
The present invention relates to a method of treating MS in a human patient in need of such treatment and comprises administering to said patient a combination therapy in a single oral dosage form (e.g. a tablet or capsule) of dim ethyifurrta rate and one agent selected from teriflunomide (or its prodrug leflunomide), fingolimod and laquinimod. This combination is more effective than the single agents alone and / or has reduced side effects and better tolerability than the single agents alone and / or can be given in a reduced frequency. Moreover, the present invention is directed to a pharmaceutical composition suitable for the oral treatment of multiple sclerosis consisting of dimethylfumarate and one agent selected from teriflunomide, fingolimod and laquinimod as active ingredients and one or more pharmaceutically acceptable excipients.
Owner:BIOGEN SWISS MFG GMBH
Novel processes for making- and a new crystalline form of- leflunomide
New leflunomide Form III is disclosed, along with processes for preparing it. The present invention also provides an economic process for preparing leflunomide Form II and a process for preparing leflunomide Form I from leflunomide Form III. Pharmaceutical compositions and dosage forms containing the new form and methods of using them for the treatment of rheumatoid arthritis are also disclosed.
Owner:AVRUTOV IIYA +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com