Leflunomide tablet for treating adult rheumatoid arthritis
A technology for rheumatoid arthritis and leflunomide tablets, applied in the field of leflunomide tablets, can solve the problems of difficulty in improving dissolution rate, affecting bioavailability and curative effect, poor water solubility of leflunomide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: the preparation of leflunomide crystal
[0014] (1) leflunomide is dissolved in the solvent of N-methylacetamide, and the required amount of solvent per g of leflunomide is 100ml; (2) after heating to 40°C for dissolution, add seed crystals after cooling to room temperature; (3) Cool to below 0°C, stir and crystallize, the crystallization temperature is -10°C, filter, dry, collect crystals to obtain leflunomide crystals.
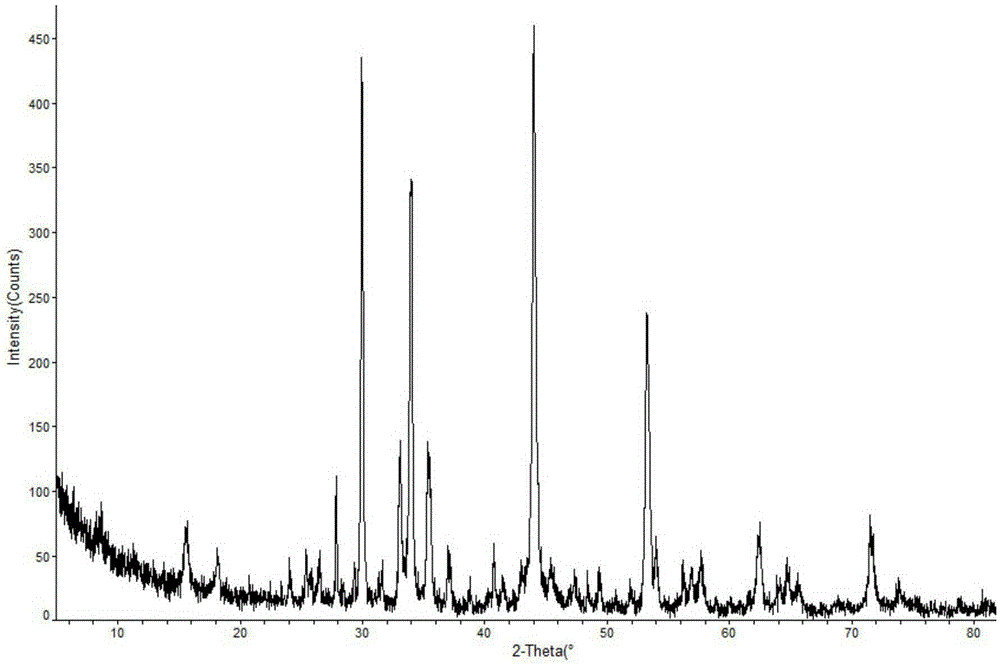
[0015] The prepared leflunomide crystals are measured by powder X-ray diffractometry, and the X-ray powder diffraction patterns represented by 2θ ± 0.2° diffraction angle are at 26.6°, 30.02°, 33.16°, 34.02°, 35.5°, 35.56°, The characteristic diffraction peaks are shown at 44.1°, 53.34° and 72.9°.
Embodiment 2
[0017] Take 10g leflunomide, 50g lactose, 40g starch, 20g hydroxypropyl cellulose, 5g sodium lauryl sulfate, 5g micropowder silica gel, 1g polyethylene glycol 6000, 4g sodium carboxymethyl starch, 0.5g stearin Magnesium acid, the preparation method is as follows: Leflunomide is pulverized, weighed and mixed with lactose, starch, sodium lauryl sulfate, polyethylene glycol 6000, and then add hydroxypropyl Appropriate amount of cellulose-based aqueous solution, making soft materials, granulating, blast drying, and granulating, adding prescription amount of magnesium stearate and micropowder silica gel, mixing, and tableting to obtain 1000 Leflunomide tablets.
[0018] Described leflunomide is a crystal, which is measured by powder X-ray diffractometry. The X-ray powder diffraction spectrum represented by 2θ ± 0.2° diffraction angle is at 26.6°, 30.02°, 33.16°, 34.02°, 35.5°, 35.56° °, 44.1°, 53.34° and 72.9° showed characteristic diffraction peaks.
Embodiment 3
[0020] Take 15g leflunomide, 40g lactose, 50g starch, 15g hydroxypropyl cellulose, 7g sodium lauryl sulfate, 3g micropowder silica gel, 3g polyethylene glycol 6000, 2g sodium carboxymethyl starch, 0.7g stearin Magnesium acid, the preparation method is as follows: Leflunomide is pulverized, weighed and mixed with lactose, starch, sodium lauryl sulfate, polyethylene glycol 6000, and then add hydroxypropyl Appropriate amount of cellulose-based aqueous solution, making soft materials, granulating, blast drying, and granulating, adding prescription amount of magnesium stearate and micropowder silica gel, mixing, and tableting to obtain 1000 Leflunomide tablets.
[0021] Described leflunomide is a crystal, which is measured by powder X-ray diffractometry. The X-ray powder diffraction spectrum represented by 2θ ± 0.2° diffraction angle is at 26.6°, 30.02°, 33.16°, 34.02°, 35.5°, 35.56° °, 44.1°, 53.34° and 72.9° showed characteristic diffraction peaks.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com