Novel environment-friendly process for preparing leflunomide
A technology for leflunomide and trifluoromethylaniline is applied in the field of green technology for preparing leflunomide, and can solve the problems of a large amount of industrial waste gas and acidic waste water, affecting the quality of leflunomide products, serious industrial pollution and the like, Achieve stable product quality, high yield and high regioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
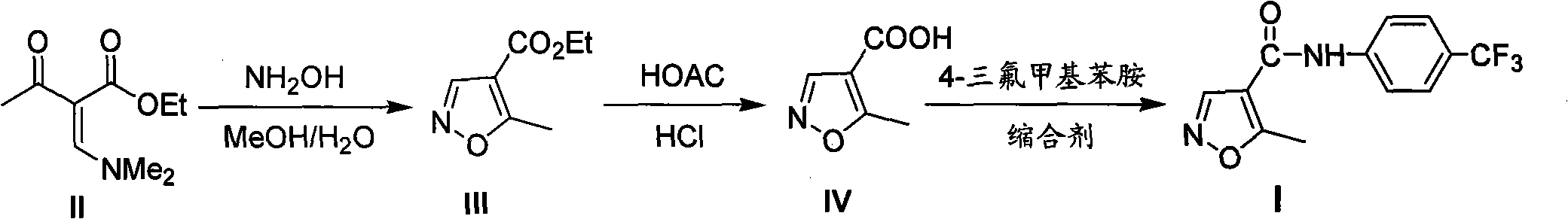
[0024] Example 1: Ethyl 5-methylisoxazole-4-carboxylate (III)
[0025] Take ethyl N,N-dimethylaminomethyleneacetoacetate (185g, 1.0mol) and add it to 600ml of methanol, start stirring, start freezing, cool the reaction system to -5°C, and slowly add 50% An aqueous solution of hydroxylamine (66 g, 1.0 mol) was added dropwise to keep the reaction system stable at -5°C to 0°C. After the dropwise addition, raise the temperature to 10°C to 15°C to continue the reaction until the raw materials disappear. After the reaction was completed, the reaction solution (200ml×3) was extracted with dichloromethane, and the organic phases were combined. The organic phase was washed once each with saturated aqueous sodium bicarbonate solution (300ml) and water (300ml), dried over anhydrous sodium sulfate, and concentrated to obtain 143g of milky white liquid with a yield of 92.2%.
Embodiment 2
[0026] Example 2: 5-Methylisoxazole-4-carboxylic acid (IV)
[0027] Add ethyl 5-methylisoxazole-4-carboxylate (155g, 1.0mol) into a mixed solvent of 36% hydrochloric acid (300ml) and glacial acetic acid (150ml), start stirring, and heat to reflux until the raw material disappears . After the reaction was completed, the solvent was evaporated to obtain a taupe solid, which was added to toluene (150ml), heated to 90°C to 100°C to dissolve, and after cooling to room temperature, solid crystals were precipitated, filtered, the filter cake was washed with toluene and water, and dried to obtain White solid 82g, yield 64.6%.
Embodiment 3
[0028] Embodiment 3: Leflunomide (I)
[0029]5-Methylisoxazole-4-carboxylic acid (127g, 1.0mol) was dissolved in dichloromethane (600ml), and after cooling to -5°C in an ice-water bath, triethylamine (106g, 1.05mol) was slowly added, Keep the temperature not exceeding 0°C. After the addition is complete, slowly add a solution of methyl chloroformate (94.5g, 1.0mol) in dichloromethane (200ml) dropwise, and keep the temperature not exceeding 0°C during the dropwise addition. After the dropwise addition was continued at 0°C for half an hour, a solution of 4-trifluoromethylaniline (169g, 1.05mol) in dichloromethane (200ml) was added dropwise, keeping the temperature below 0°C during the dropwise addition. After the dropwise addition, continue to react at -5°C to 0°C until the raw materials disappear. After the reaction was completed, the reaction solution was washed with aqueous sodium bicarbonate solution (300ml×2), water (300ml×2), and saturated brine (300ml×2), and the organic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com