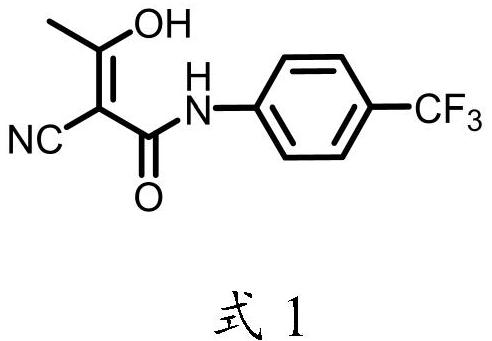
Simple preparation method of teriflunomide
A kind of teriflunomide, simple technology, applied in the field of preparation of teriflunomide, can solve the problem of low yield of end product teriflunomide and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The invention provides a kind of simple and convenient preparation method of teriflunomide, comprises the following steps:
[0043] (1) condensing 5-methylisoxazole-4-carboxylic acid and a condensing agent in a solvent under alkaline conditions to obtain an active ester system;
[0044] (2) Condensing the active ester system and 4-trifluoromethylaniline in a solvent to obtain the intermediate leflunomide;
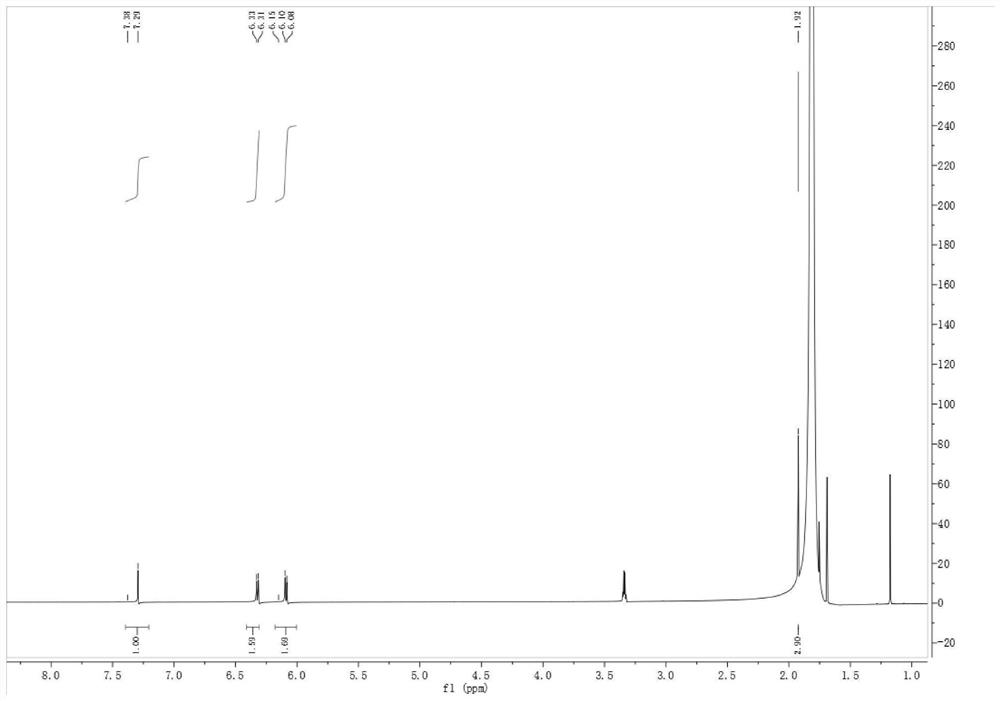
[0045] (3) The obtained intermediate leflunomide is subjected to alkali treatment and acid treatment in sequence to obtain teriflunomide.
[0046] In step (1) of the present invention, the solvent is acetone, acetonitrile, dichloromethane, chloroform, carbon tetrachloride, ethyl acetate, toluene, benzene, xylene, N,N-dimethylformamide, di One or more of methyl sulfoxide, diethyl ether, tetrahydrofuran and dioxane, preferably dichloromethane or N,N-dimethylformamide.
[0047] In step (1) of the present invention, the condensing agent is carbonyl imidazole, benzotria...
Embodiment 1
[0064] (1) Preparation of active ester
[0065] 50g (0.39mol) of 5-methylisoxazole-4-carboxylic acid was dissolved in 500ml of dichloromethane and placed in an ice bath; 245g of 2-(7-azabenzotriazole)-N,N,N' , N'-tetramethyluronium hexafluorophosphate (0.65mol) was added to the above reaction system and 100.81g (0.78mol) N,N-diisopropylethylamine was added, and the active ester was obtained after stirring in an ice bath for 1 hour , this step does not require purification and post-treatment, and is directly used in the next reaction.
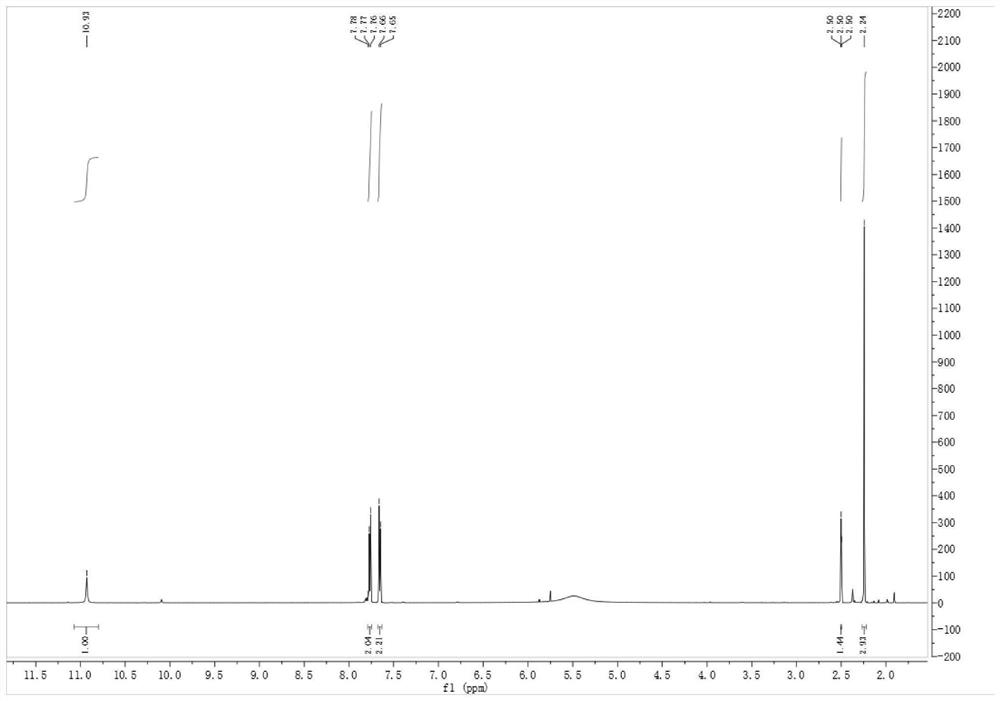
[0066] (2) Preparation of intermediate leflunomide
[0067] Add 100 g (0.62 mol) of 4-trifluoromethylaniline into the above-mentioned active ester reaction system, stir at 0°C for 3 h, and track the reaction by TLC until the reaction of the raw materials is complete. After the reaction, the reaction solution was poured into 1 L of ice water and stirred for 30 min, a large amount of white solid was precipitated, filtered, the filter cake was wa...
Embodiment 2
[0073] (1) Preparation of active ester
[0074] 50g (0.39mol) of 5-methylisoxazole-4-carboxylic acid was dissolved in 500ml of methylene chloride and placed in an ice bath; 368.4g (0.65mol) of hexafluorophosphate benzotriazol-1-yl-oxyl Tripyrrolidinylphosphine was added to the above reaction system and 78.93g (0.78mol) of triethylamine was added. After stirring in an ice bath for 1 hour, the active ester was obtained. This step did not require purification and post-treatment, and was directly used in the next reaction.
[0075] (2) The preparation of intermediate leflunomide is the same as in Example 1
[0076] (3) Preparation of Teriflunomide
[0077] Suspend 50 g (0.19 mol) of the intermediate leflunomide in 500 mL of water, add 74.07 g (1.32 mol) of potassium hydroxide to react until clarified, then react at room temperature for 2 h, and track the reaction to completion by TLC. After the reaction, 1N hydrochloric acid was added dropwise to the reaction solution to adjust ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com