Novel crystal form compound of leflunomide and preparation method of novel crystal form compound
A leflunomide and compound technology, applied in the field of pharmaceutical compound preparation, can solve the problem of not giving a spectrum, and achieve the effects of good reproducibility and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of Leflunomide Form I (refer to CN107311954A).
Embodiment 2
[0041] Example 2: Preparation of leflunomide crystal form II (refer to CN107311954).
Embodiment 3
[0042] Example 3: Preparation of Leflunomide Form V.
[0043] A preparation method for new crystal form V of leflunomide, comprising the following steps:
[0044] A. Heating leflunomide at a uniform speed until the raw material of leflunomide is completely melted and then vaporized (180-200°C);
[0045] B, cooled to room temperature;
[0046] C. Collect the sample after desublimation of the upper layer of glass.
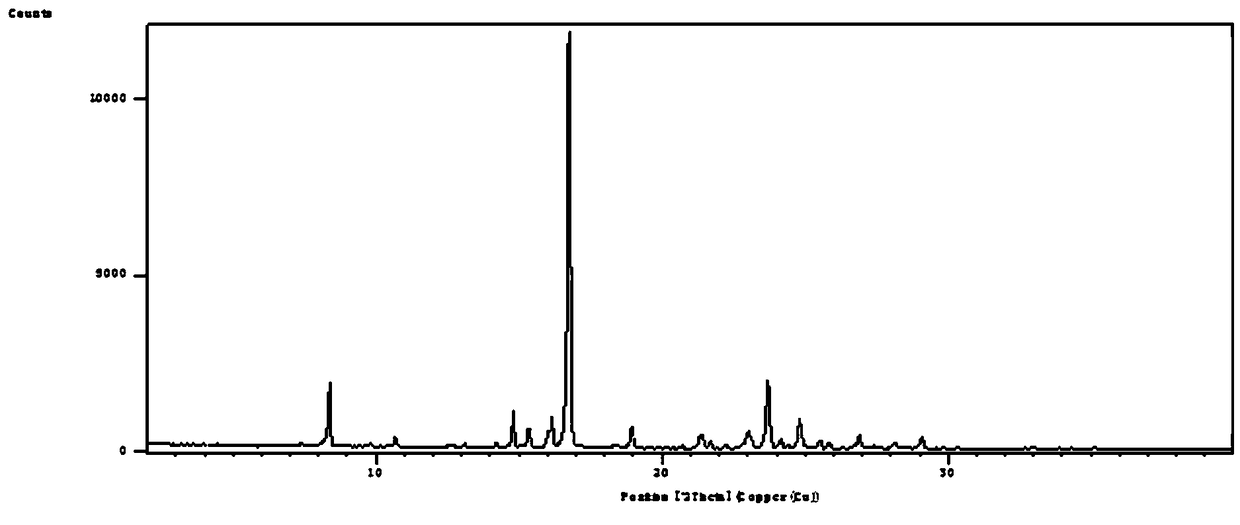
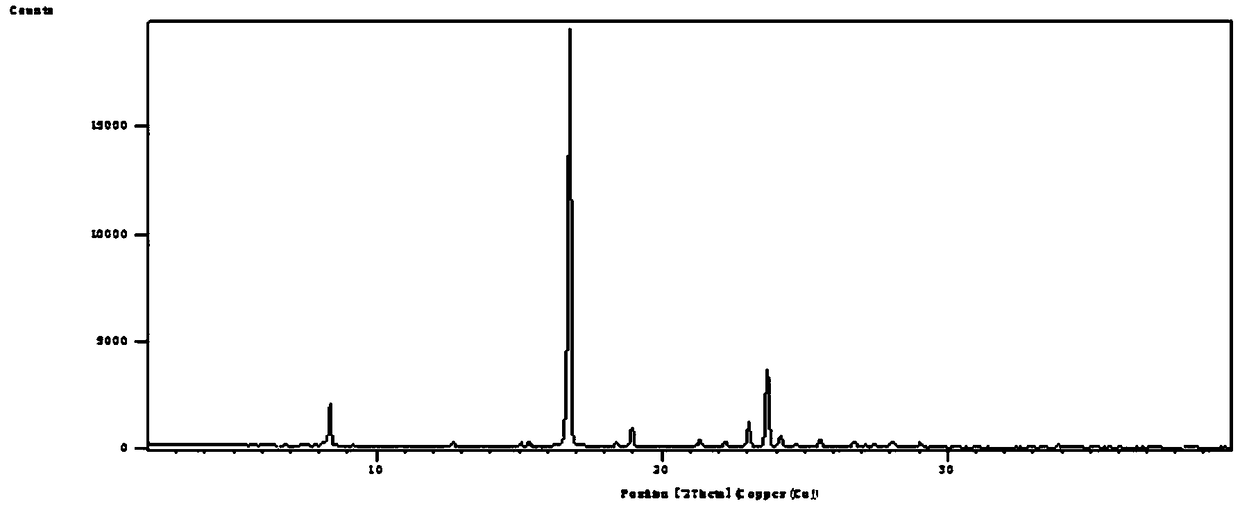
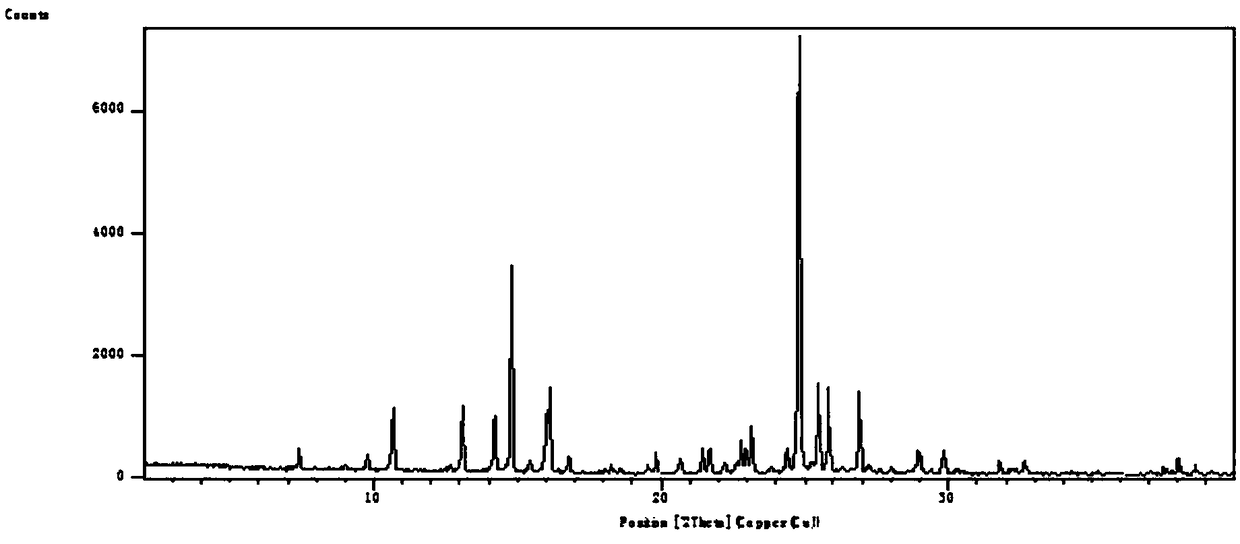
[0047] Leflunomide Form V has the following characteristics:
[0048] 1. Powder X-ray Diffraction
[0049] Instrument: Sharp Shadow X-ray Diffractometer (PANalytical, Netherlands)
[0050] Target: Cu-Kα radiation
[0051] wavelength:
[0052]X-ray light tube voltage: 45kV
[0053] X-ray tube electric current: 40mA
[0054] Step size: 0.01313°
[0055] Scanning speed: 0.041683° / s
[0056] Scanning range: 2°-40°
[0057] The results show that: at 2θ7.8255°, 8.7099°, 9.1789°, 9.6017°, 11.1488°, 14.9436°, 15.6434°, 18.6293°, 19.3055°, 20.2744°, 23.3166°, 23....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com