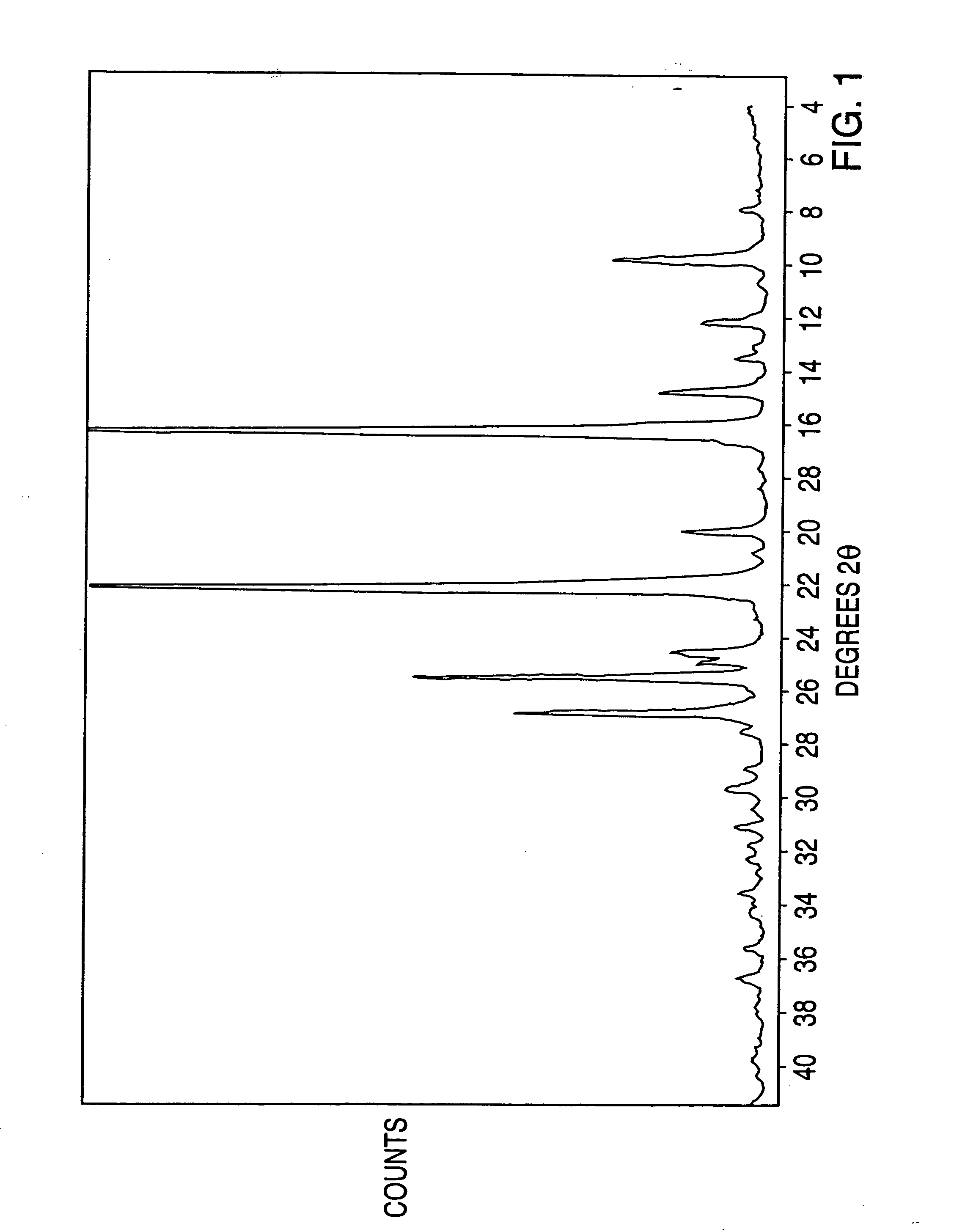
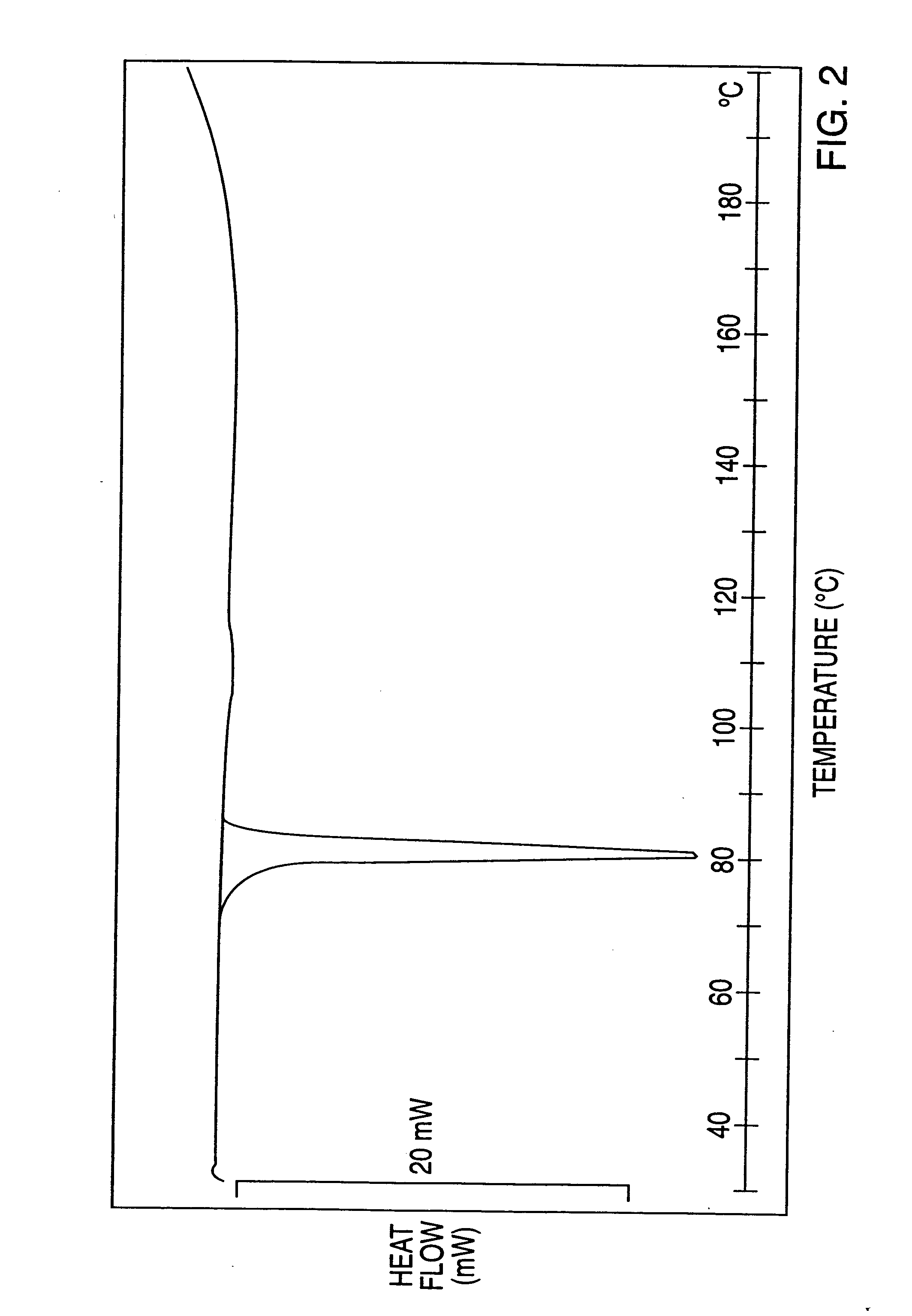
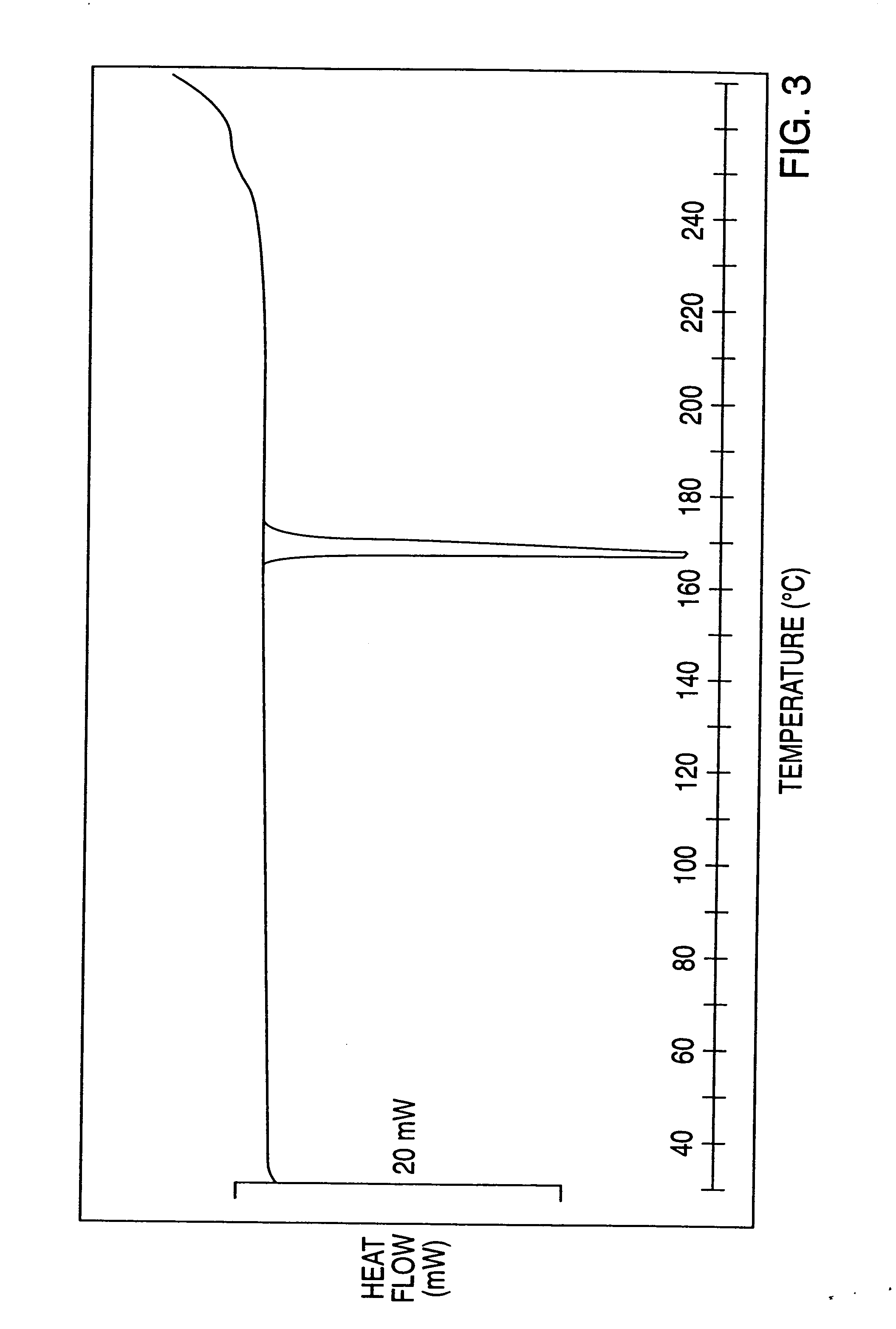
Novel processes for making- and a new crystalline form of- leflunomide
a technology of leflunomide and crystallization process, which is applied in the field of new crystalline form of leflunomide, can solve the problems of increasing cost, time-consuming method, and cost of industrial production of pharmaceuticals, and achieves the effects of high crystallization rate, easy recovery and recycling, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Crystallization of Form II from DMSO
DMSO (8 ml) was warmed to 50° C. Leflunomide (5.6 g) was added to the DMSO with stirring. After complete dissolution of the leflunomide, the stirred solution was allowed to cool to ambient temperature. Crystal formation was noted when the solution temperature reached 40° C. The mixture was stirred for another 30 minutes and then the crystals were isolated by filtration, washed with water and then dried under vacuum at 30° C. to give leflunomide Form II (3.4 g, 61%).
example 2
Crystallization of Form II from DMSO / Water
Leflunomide (1.07 g) was dissolved in stirred DMSO (6 ml) at ambient temperature. Water (12 ml) was added dropwise to the stirred solution. The mixture was stirred for 30 minutes. The crystals which formed were isolated by filtration, washed with water and then dried under vacuum at room temperature to give leflunomide Form II (1 g, 93%).
example 3
Crystallization of Form II from DME / Hexane
Leflunomide (6.1 g) was dissolved in DME (10 ml) at ambient temperature. Hexane (16 ml) was added to the stirred solution. The mixture was then stirred for about 30 min. after which time crystallization appeared to have ceased. The crystals were isolated by filtration through a paper filter and dried under vacuum at 30° C. to give leflunomide Form II (2.4 g, 39%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| dissolution temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com