Crystal form compound of leflunomide, and preparation method and application thereof
A technology of leflunomide and its compounds, which is applied in the field of pharmaceutical crystal forms, can solve problems affecting the stability and instability of preparations, and affect the production quality of raw materials and preparations, and achieve good preparation processability, good selection, and stable properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Heat and dissolve 8 g of leflunomide bulk drug (crystalline form I) in 10 ml of the following solvents, cool to room temperature (about 22° C.), and detect the obtained solid by X-ray powder diffraction. Form IV was obtained in PEG400, as shown in Table 2 below.
[0069] Table 2
[0070] Numbering
Embodiment 2
[0071] Embodiment 2 Preparation of Leflunomide Form IV
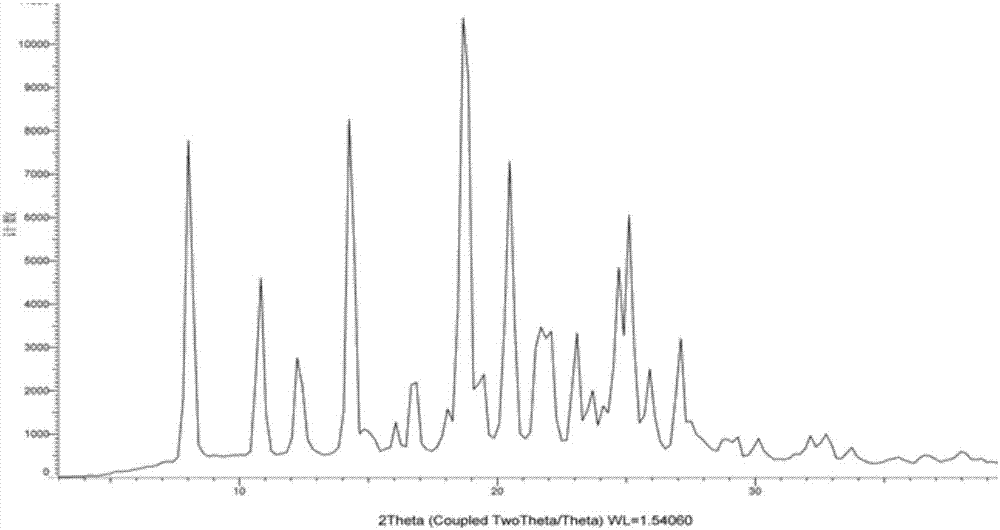
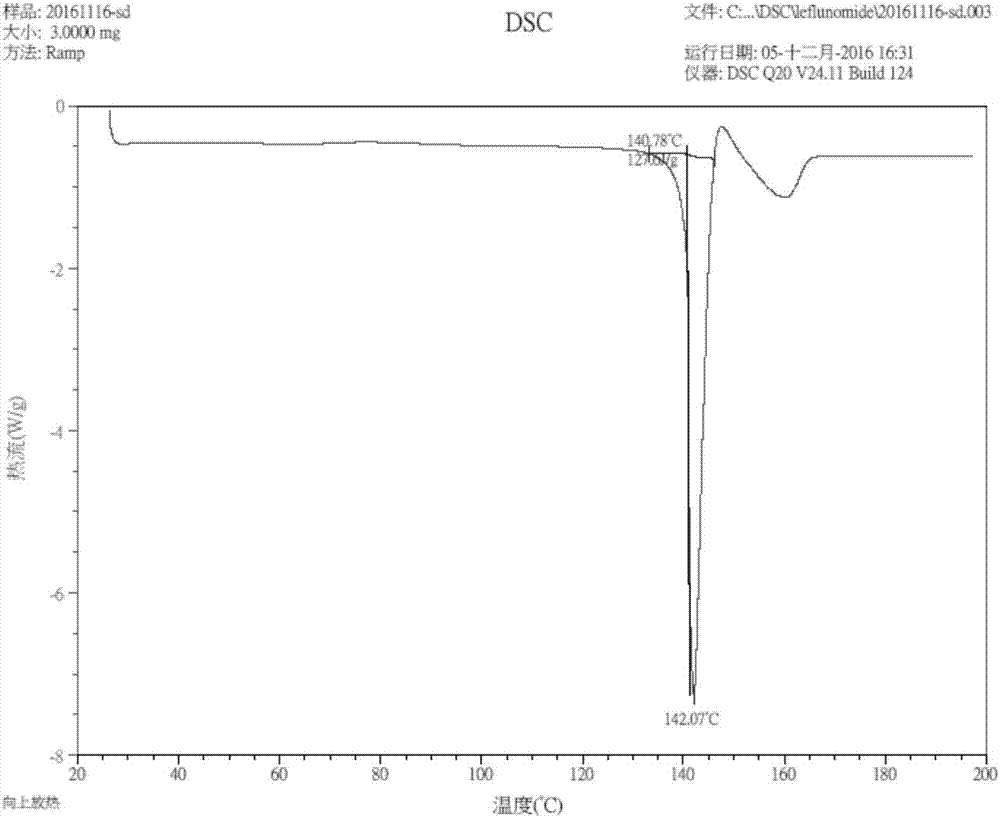
[0072] Suspend 8g of leflunomide raw material (form I) in 10ml of PEG 400, heat to 68°C, and completely dissolve leflunomide, stir for 10 hours, cool to room temperature (22°C), and filter to obtain a solid , washed with water for 2-5 times, dried to obtain crystals after washing, X-ray powder diffraction detection and DSC detection, the crystals are crystal form IV, see figure 1 .
Embodiment 3
[0073] Embodiment 3 Preparation of Leflunomide Form IV
[0074] Suspend 1 g of leflunomide raw material (form I) in 5 g of PEG 200, heat to 68°C, and completely dissolve leflunomide, stir for 36 hours, cool to room temperature (22°C), and filter to obtain a solid , washed with water for 2-5 times, dried to obtain crystals after washing, X-ray powder diffraction detection and DSC detection were performed, and the crystals were crystal form IV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com