Preparation method of teriflunomide
A technology for teriflunomide and trifluoromethylaniline, which is applied in the field of preparation of teriflunomide, can solve the problems of poor stability of cyanoacetyl chloride, serious pollution of thionyl chloride, unsuitable for industrialized production and the like, and achieves easy operation. , the effect of low cost and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
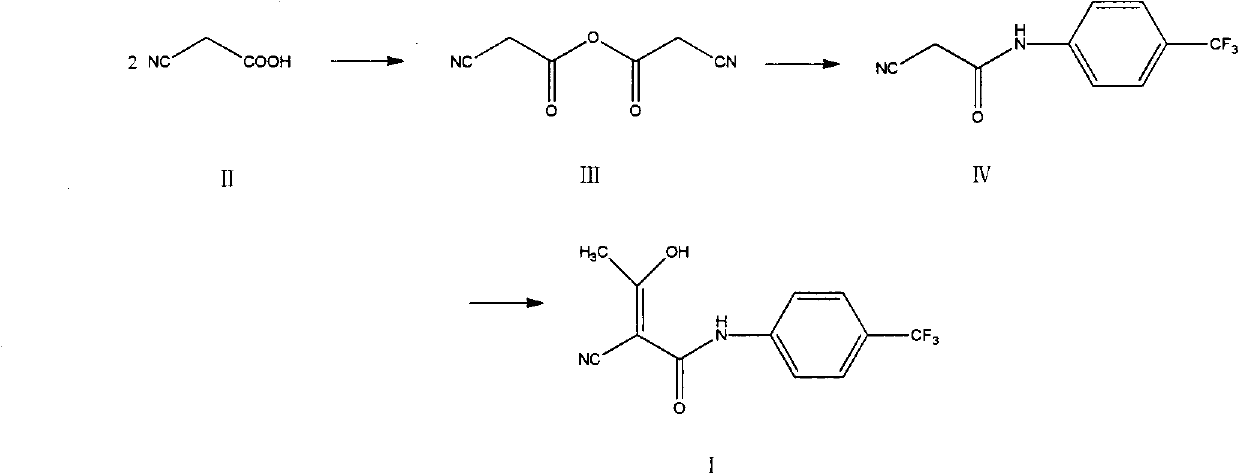
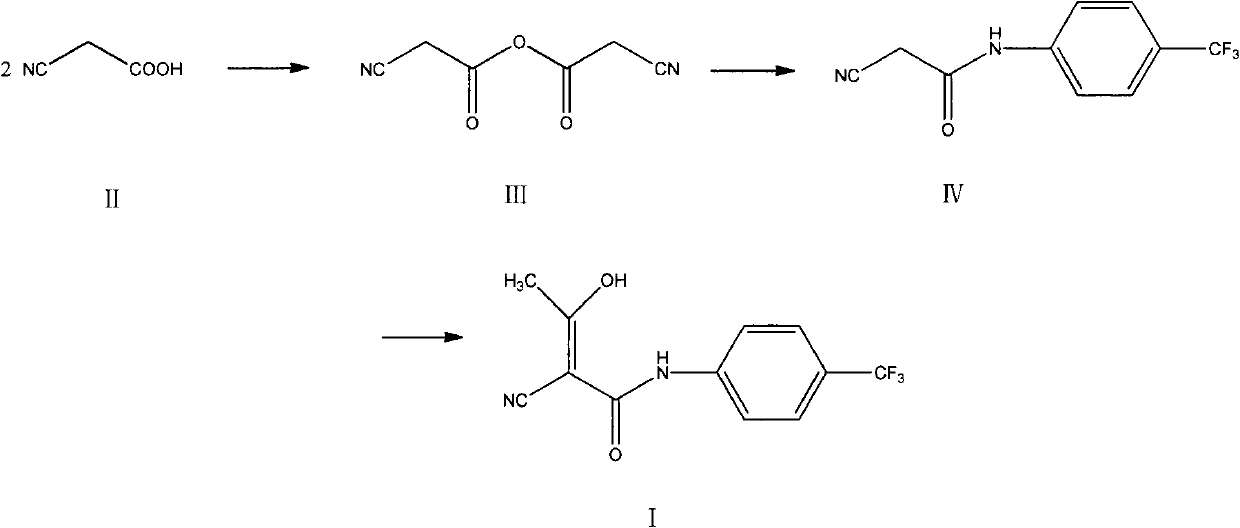
[0036] Embodiment 1, the preparation of cyanoacetic anhydride (III)
[0037] ①Toluene water separation preparation
[0038] Add 34.0 g of cyanoacetic acid (II) and 200 ml of toluene into the reaction flask, stir, reflux and divide water, react for 16 hours, concentrate under reduced pressure, add 500 ml of acetonitrile, stir, and suction filter, and the filtrate is concentrated under reduced pressure to obtain a brownish-red liquid (III) 25.2 g, yield 82.9%.
[0039] ②Phosphorus pentoxide dehydration preparation
[0040] Add 34.0g of cyanoacetic acid (II), 35.5g of phosphorus pentoxide, and 200ml of anhydrous tetrahydrofuran into the reaction flask, raise the temperature to 65°C, stir for 24h, suction filter while hot, rinse the filter cake with a small amount of acetonitrile, and concentrate the filtrate under reduced pressure 27.3 g of brown-red liquid was obtained, with a yield of 89.8%.
Embodiment 2
[0041] Embodiment 2, the preparation of 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide (IV)
[0042] Add 22.8g of cyanoacetic anhydride (III), 12.08g of p-trifluoromethylaniline, 120ml of acetonitrile, 0.075g of concentrated sulfuric acid into the reaction flask, heat up to 81°C, stir for 36h, concentrate under reduced pressure, add 300ml of ethyl acetate, and stir , the resulting solution was washed with 150ml×3 of 2.7mol / L hydrochloric acid solution, then washed with 150ml×3 of saturated aqueous sodium chloride solution, the organic layer was concentrated under reduced pressure, and the residue was recrystallized with ethanol to obtain 12.5g of light yellow powder solid (IV) , mp 191-193°C, yield 73.1%.
[0043] MS (m / z): 227 (M + ).
Embodiment 3
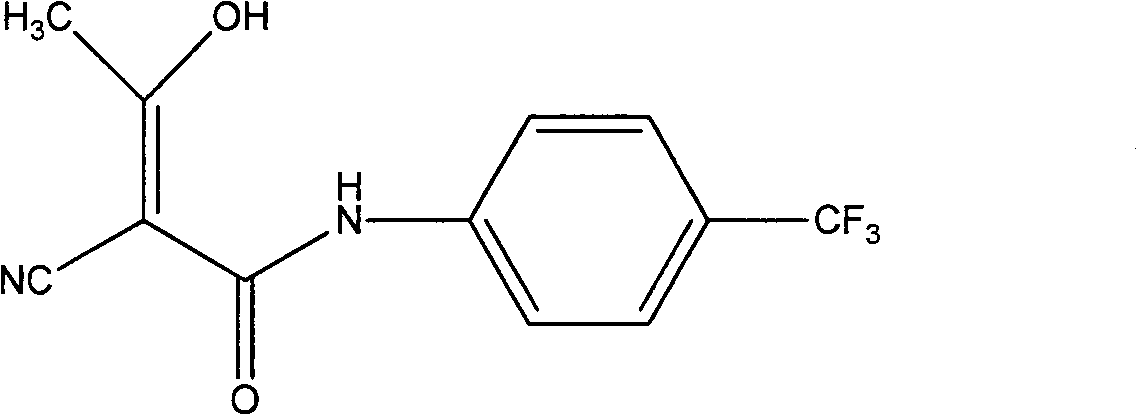
[0044] Embodiment 3, the preparation of teriflunomide (I)
[0045] Add 6.0 g of sodium hydride and 100 ml of dry tetrahydrofuran into the reaction flask, and add dropwise 25 ml of tetrahydrofuran solution containing 11.4 g of 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide (IV) in an ice bath , stirred for 1 h, added 4.3 g of acetyl chloride dropwise, reacted at room temperature for 24 h, quenched with acetic acid, concentrated under reduced pressure, added 200 ml of water, stirred, washed the solution with 100 ml of dichloromethane × 3, adjusted the pH of the aqueous layer to 2 with 10% hydrochloric acid, A large amount of solid was precipitated, filtered with suction, and the filter cake was recrystallized with methanol to obtain 10.6 g of white solid (I), mp 228-230°C, yield 78.5%.
[0046] 1 H-NMR (DMSO-d 6 )δ: 7.72 (d, 2H), 7.59 (d, 2H), 2.13 (s, 3H), 11.67 (s, 1H).
[0047] MS (m / z): 269 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com