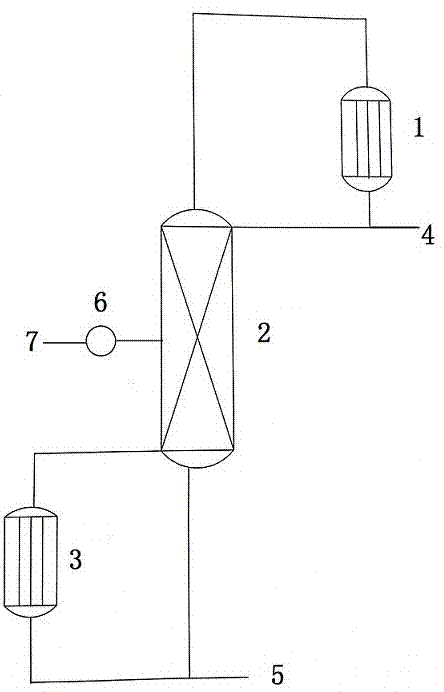
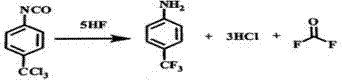
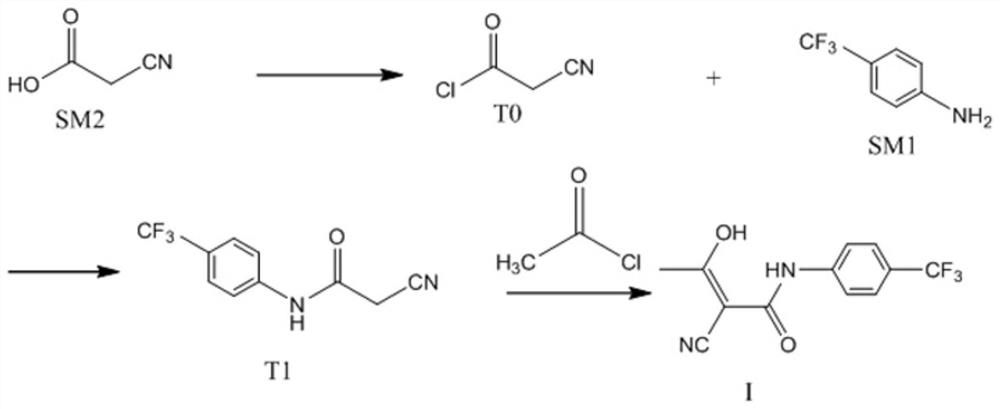
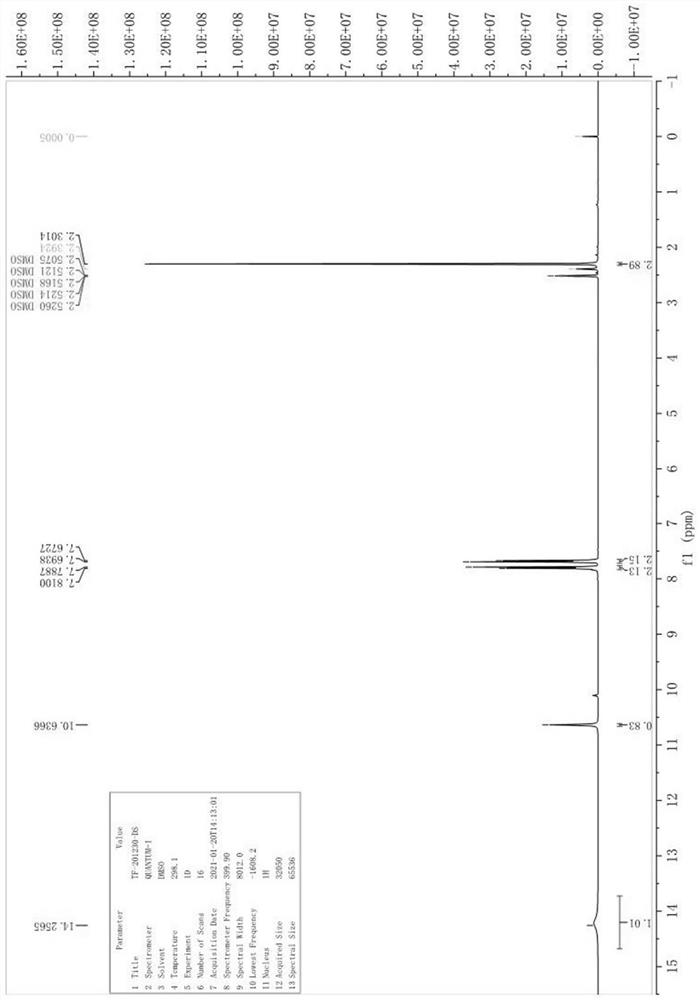
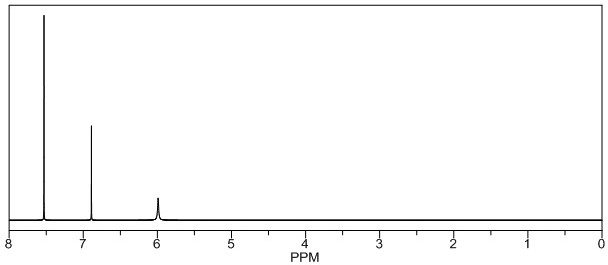
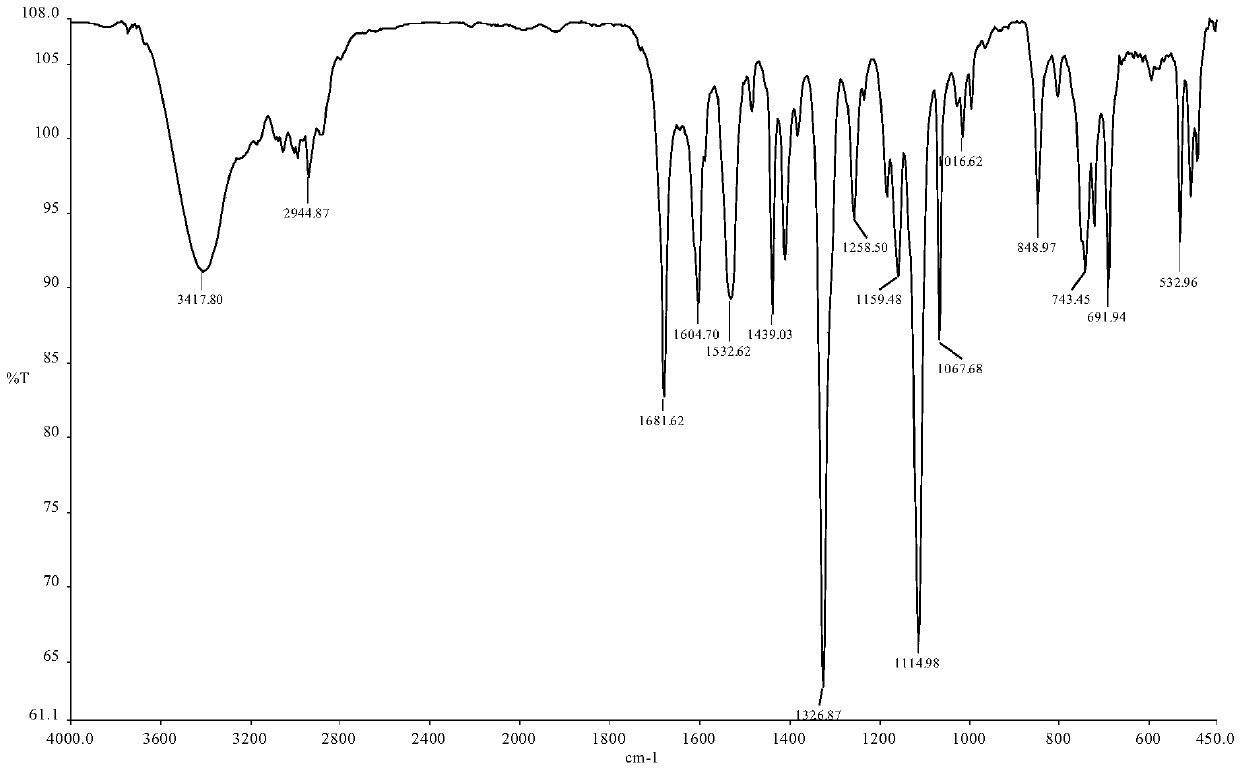
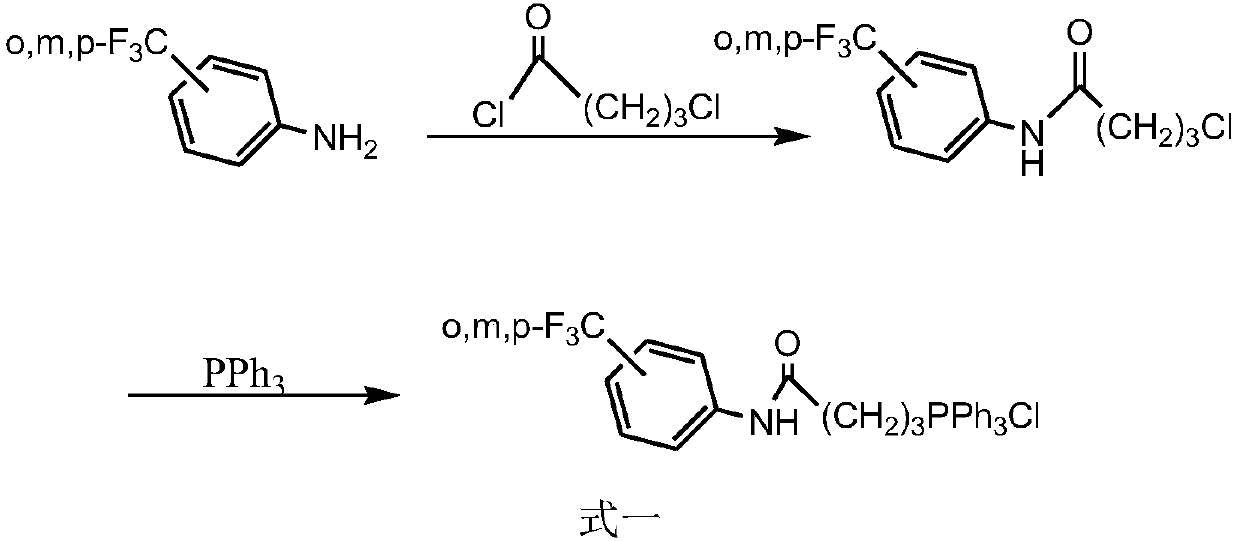
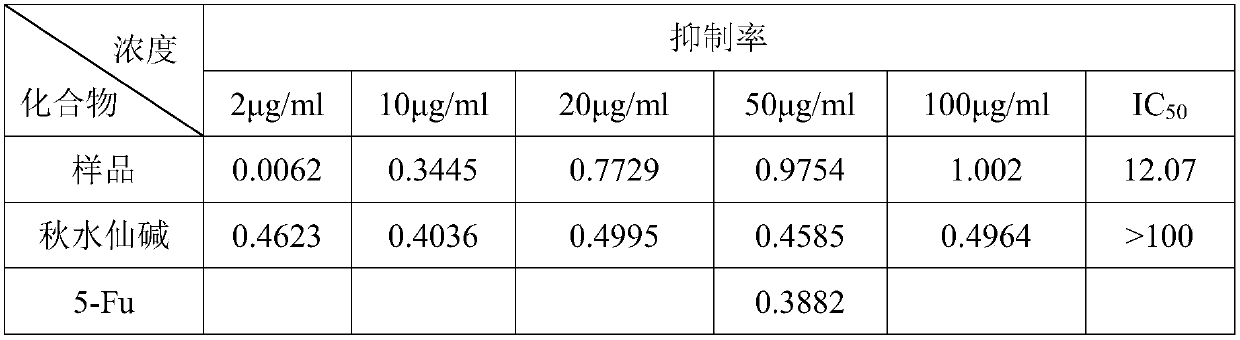
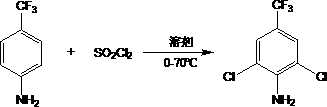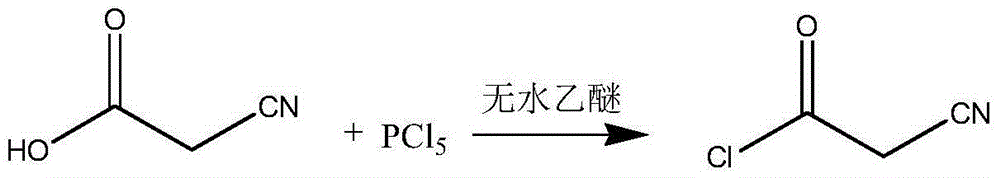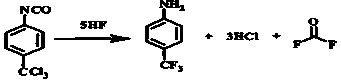Patents
Literature
31 results about "Para-trifluoromethylaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
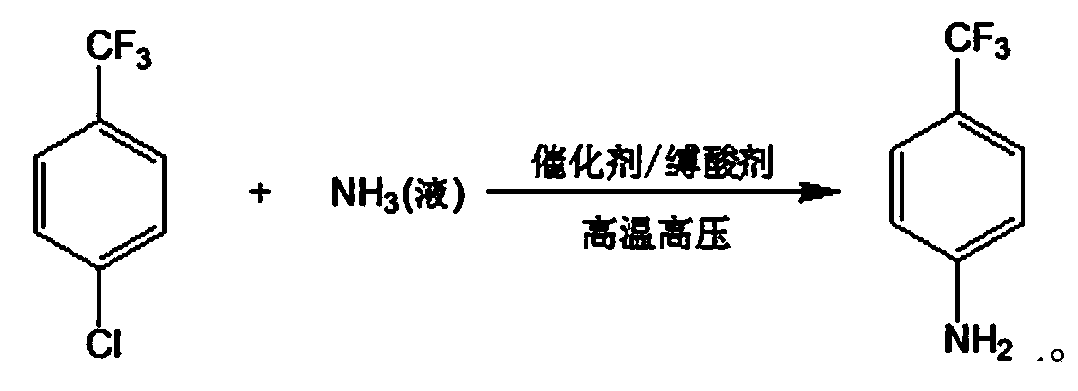
Production method of p-trifluoromethylaniline
InactiveCN102643202APhysical/chemical process catalystsOrganic compound preparationP-chlorobenzotrifluorideAlcohol
The invention discloses a production method of p-trifluoromethylaniline. The production method comprises the following step: enabling p-chlorobenzotrifluoride and liquid ammonia to react in the presence of alcohol and a catalyst to get the p-trifluoromethylaniline, wherein the catalyst mainly comprises cuprous chloride and potassium fluoride. According to the production method disclosed by the invention, the high-efficient compound catalyst is adopted, and the yield can achieve 90%; and an organic solvent in the production method can be applied mechanically in a circulating manner, no wastewater exists, the excess liquid ammonia can be produced into ammonia water for selling, and the catalyst can be recycled.
Owner:江苏优普生物化学科技股份有限公司
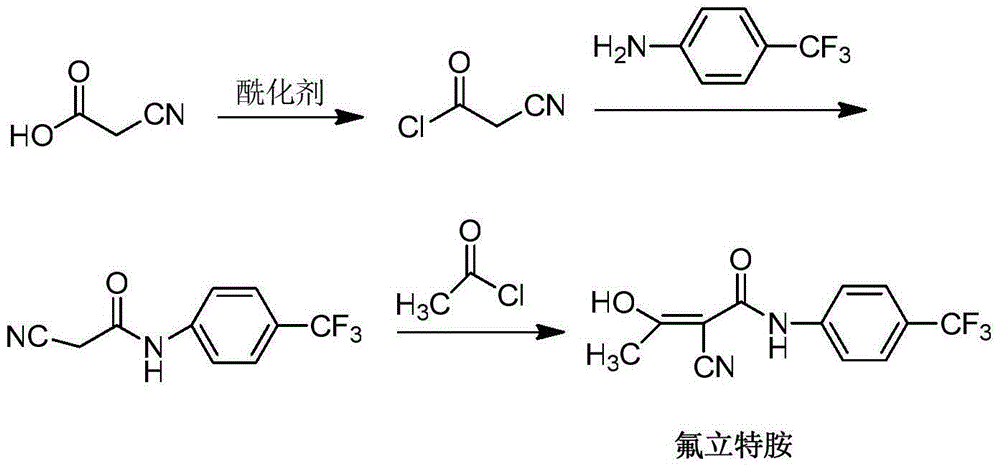
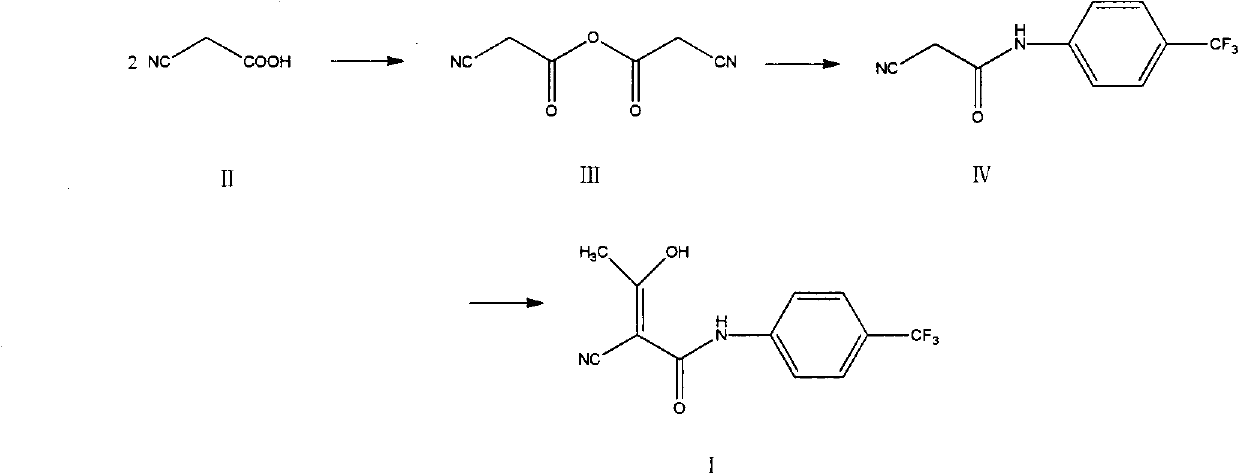
Preparation method of teriflunomide
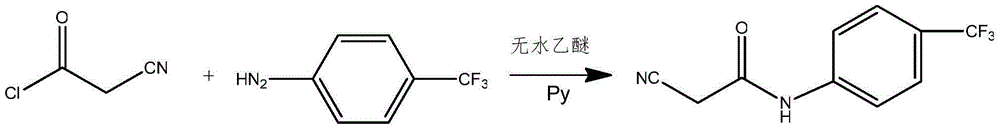
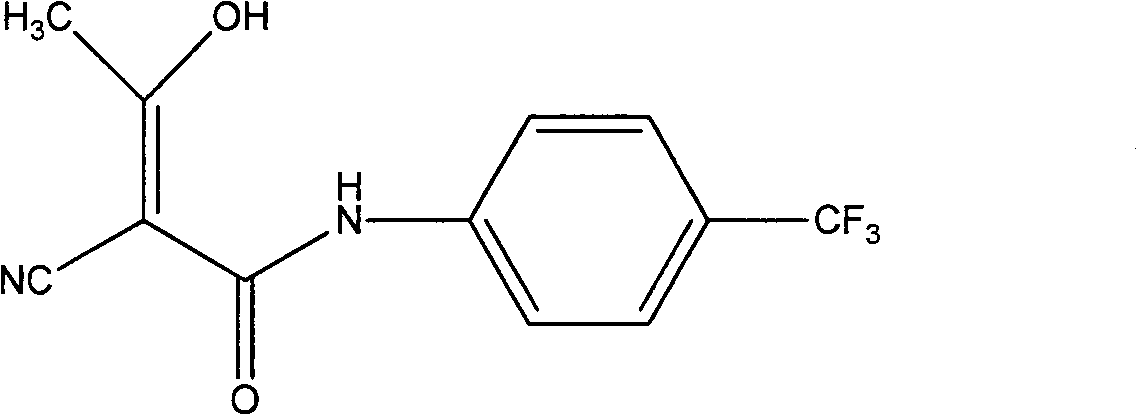
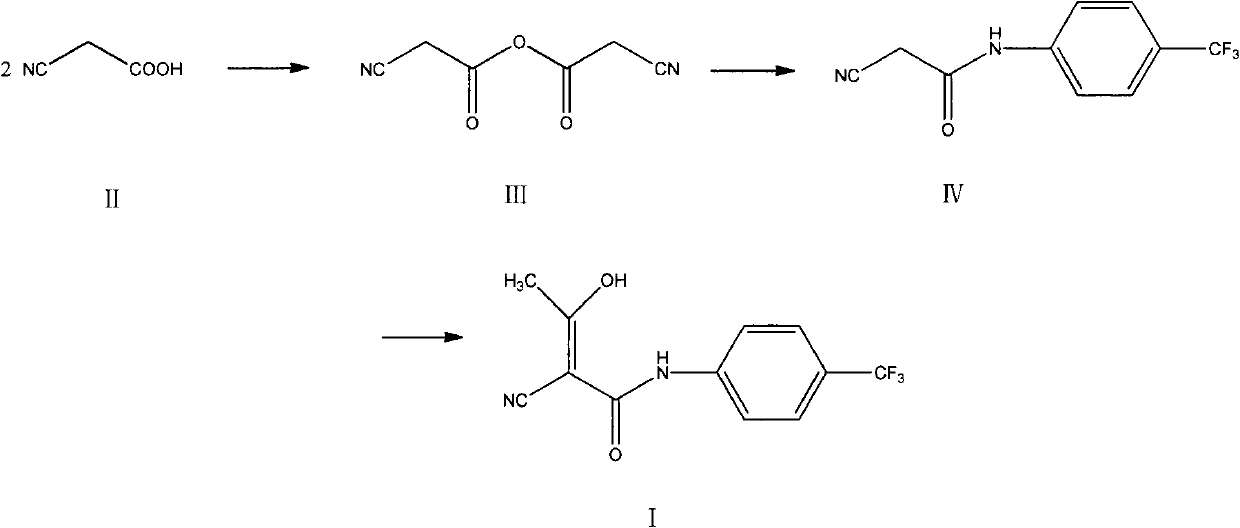
InactiveCN102786437AImprove water stabilitySimple and fast operationCarboxylic acid nitrile preparationOrganic compound preparationAcetic anhydrideCyanoacetic acid
The invention relates to a preparation method of (Z)-2-cyano-3-hydroxy-N-(4-(trifluoromethyl)phenyl)-2-butenamide (I). The preparation method is characterized in that cyanoacetic acid (I) is dehydrated and condensed to obtain cyan acetic anhydride (III), the cyan acetic anhydride (III) reacts with trifluoromethyl phenyl under the action of catalyst to obtain 2-cyano-N-(4-trifluoromethyl-phenyl)-ethanamide (IV), and the 2-cyano-N-(4-trifluoromethyl-phenyl)-ethanamide (IV) reacts with the acetylchloride in the presence of sodium hydride to obtain teriflunomide (I).
Owner:CHINA PHARM UNIV
Production process of p-trifluoromethyl aniline
ActiveCN1847214AOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingPotassium fluorideAniline
The present invention discloses the production process of p-trifluoromethyl aniline. P-trifluorotoluene and liquid ammonia are reacted in the presence of alcohol and catalyst to obtain p-trifluoromethyl aniline. The catalyst may consist of ferrocene, triphenyl phosphor, potassium fluoride, ammonium tetrabutyl bromide. The present invention has high efficient composite catalyst used, single pass conversion rate up to 66 % and yield up to 90 %. The organic solvent may be used circularly, the excessive liquid ammonia may be prepared into ammonia water, the catalyst may be regenerated and reused, and the process has no waste water exhausted.
Owner:江苏优普生物化学科技股份有限公司
Method for preparing 2,6-dichloro-4-trifluoromethyl phenylamine
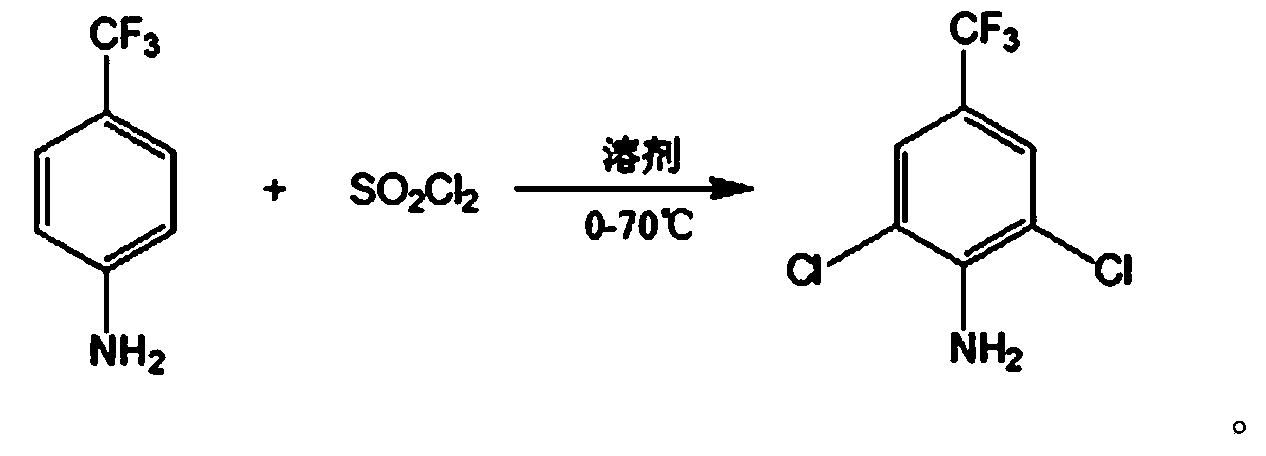
InactiveCN103408437ASimple processEasy to operateOrganic compound preparationAmino compound preparationSulfonyl chlorideReaction temperature
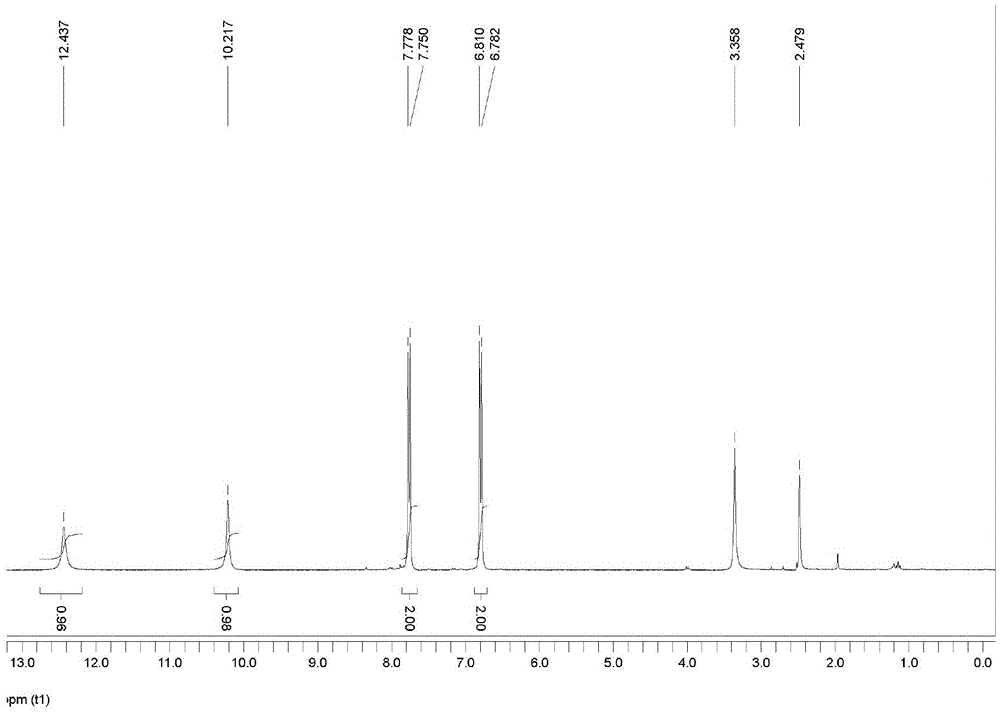
The invention relates to a method for preparing 2,6-dichloro-4-trifluoromethyl phenylamine. The method is characterized by comprising the following steps of: adding p-trifluoromethylaniline into a corresponding solvent; dripping a certain amount of sulfonyl chloride by controlling a reaction temperature of 0 DEG C to 70 DEG C; contiguously reacting for 1 to 15 hours by controlling the reaction temperature to 30 DEG C to 70 DEG C after the dripping of the sulfonyl chloride; removing an inorganic salt from the mixture in a water washing manner after the reaction; and performing decompression and rectification after the concentration of an organic phase, thereby obtaining the corresponding 2,6-dichloro-4-trifluoromethyl phenylamine, wherein the adopted solvent is a mixed solvent selected from any one or two and more than two materials from N,N-dimethyl formamide, ethyl acetate, dichloromethane, trichloromethane, carbon tetrachloride and dichloroethane. The method has the advantages of being simple in process, easy to operate, short in reaction time, high in yield, low in production cost and easy in industrial production. Thus, the method is an environment-friendly chemical process which has a good industrial application prospect.
Owner:JIANGSU FENGHUA CHEM IND
Synthetic method of p-trifluoromethylaniline
InactiveCN101298421ALow priceNo emissionsPhysical/chemical process catalystsOrganic compound preparationOrganic solventP-chlorobenzotrifluoride
The invention relates to a synthetic method of p-trifluoromethylaniline; p-chlorobenzotrifluoride and liquid ammonia generate an ammonolysis reaction to generate the p-trifluoromethylaniline under the action of a catalyst and an acid-binding agent; according to portion by weight, the components of the catalyst is: cuprous chloride of 1 to 10 portions, potassium fluoride of 2 to 30 portions and phase transfer catalyst of 5 to 30 portions; the adding amount of the catalyst is 8 to 70 percent of the p-chlorobenzotrifluoride (mass); the synthetic method of the invention adopts an effective compound catalyst to provide a synthetic scheme which has higher one way conversion rate and is economically practical in industry for the synthesis of the p-trifluoromethylaniline; the price of the catalyst used in the synthetic process is lower; furthermore, an organic solvent and the phase transfer catalyst can be recycled and reused; the manufacture cost is low; no waste water is discharged during the whole technique process; and the synthetic method of the invention is friendly to the environment.
Owner:太仓中化环保化工有限公司
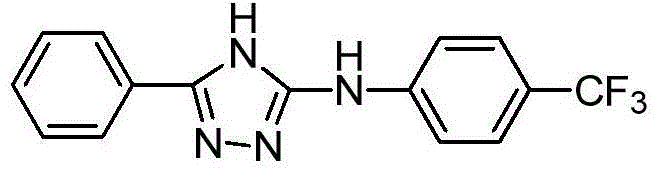
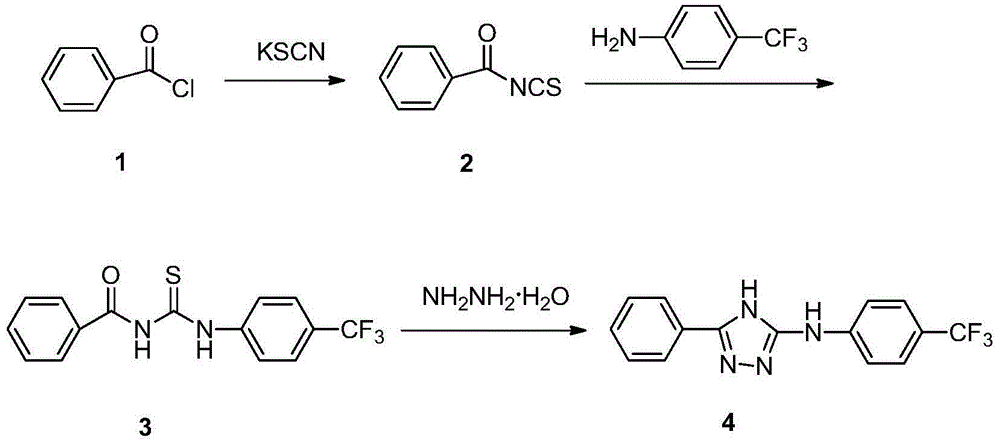
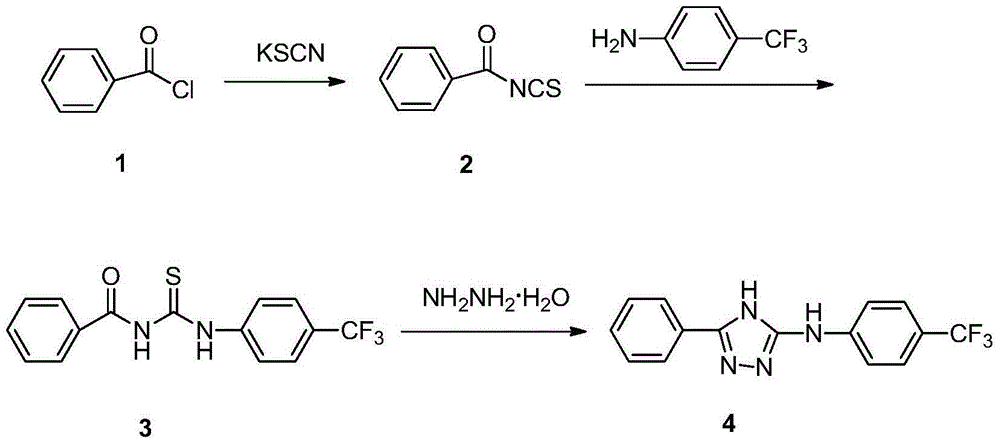
3-phenyl-5-(4-trifluoromethyl phenylamino)-4H-1,2,4-triazole as well as synthetic process and application thereof
The invention relates to 3-phenyl-5-(4-trifluoromethyl phenylamino)-4H-1,2,4-triazole as well as a synthetic process and an application thereof. A synthetic process of 3-phenyl-5-(4-trifluoromethyl phenylamino)-4H-1,2,4-triazole comprises the following steps: 1) carrying out reaction on benzoyl chloride which is taken as a raw material and potassium thiocyanate to generate benzoyl isothiocyanate; 2) carrying out reaction on benzoyl isothiocyanate and p-trifluoromethyl phenylamine to produce an intermediate 1-benzoyl-3-(4-trifluoromethyl phenylamino) thiourea; and 3) carrying out reaction on the intermediate 1-benzoyl-3-(4-trifluoromethyl phenylamino) thiourea and hydrazine hydrate to produce a target product 3-phenyl-5-(4-trifluoromethyl phenylamino)-4H-1,2,4-triazole. The 3-phenyl-5-(4-trifluoromethyl phenylamino)-4H-1,2,4-triazole has the beneficial effects that a new compound is determined by adopting a spray method when concentration of a target compound is 200ppm, the efficiency of protecting malt in 24 hours is 60%, the efficiency of protecting malt in 48 hours is 70%, and the 3-phenyl-5-(4-trifluoromethyl phenylamino)-4H-1,2,4-triazole has a potential of being further developed into an insecticide.
Owner:南雄汇星化工科技有限公司
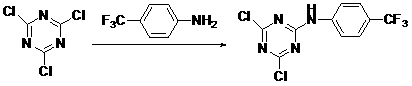
2,4-dichloro-6-(4-aminobenzotrifluoride)-1,3,5-triazine and preparation method and application thereof
The invention relates to 2,4-dichloro-6-(4-aminobenzotrifluoride)-1,3,5-triazine and a preparation method and application thereof. The structural formula is shown as follow. The preparation method includes adding cyanuric chloride and acetone solvent into a reactor, dropping aminobenzotrifluoride containing acetone solvent into the reactor when stirring, reacting for 1-3 hours at the temperature of -5-5 DEG C according to the mole ratio, 1:1-1.2 of the aminobenzotrifluoride to the cyanuric chloride, adding alkaline solution to adjust the pH (potential of hydrogen) value of the system to be 5-7 after reaction, precipitating and recrystallizing to obtain the 2,4-dichloro-6-(4-aminobenzotrifluoride)-1,3,5-triazine. The 2,4-dichloro-6-(4-aminobenzotrifluoride)-1,3,5-triazine can be applied to germicide for preventing mycosphaerella arachidicola of lawns and cotton wilt fusarium.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
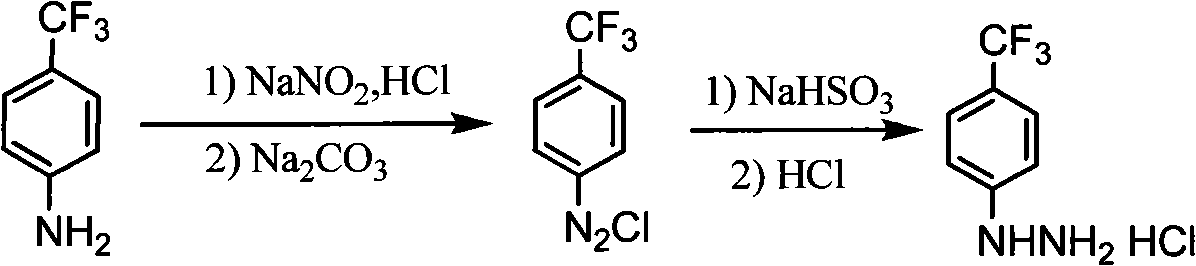
Synthesis method of para-trifluoromethyl phenyl hydrazine hydrochloride
The invention relates to a synthesis method of para-trifluoromethyl phenyl hydrazine hydrochloride, which comprises the following steps: firstly leading para-trifluoromethylaniline and sodium nitrite to carry out diazotization reaction: adding concentrated hydrochloric acid and water into a four-neck flask, and starting stirring; dripping the para-trifluoromethylaniline, generating a larger number of white solids, and controlling the temperature at -5 DEG C-15 DEG C; reducing the temperature to -5 DEG C-5 DEG C, dripping sodium nitrite solution, controlling the temperature at -5 DEG C-15 DEG C, dripping 10-12% of sodium carbonate solution, and adjusting the pH value of diazotization reaction solution to 5-7; further carrying out reduction reaction: adding sodium sulfite solution into the four-neck flask, reducing the temperature to 0-20 DEG C and starting stirring; dripping the diazotization reaction solution into the sodium sulfite solution in batches, keeping the temperature at 0-25 DEG C, and stirring at the room temperature for 1-3h; adding the concentrated hydrochloric acid, and carrying out heating reflux for 1-4h; and reducing the temperature to 0 DEG C-20 DEG C, filtering, drying, and obtaining the para-trifluoromethyl phenyl hydrazine hydrochloride. The yield is greater than 75% and the purity is 97%-99%.
Owner:大连凯飞精细化工有限公司
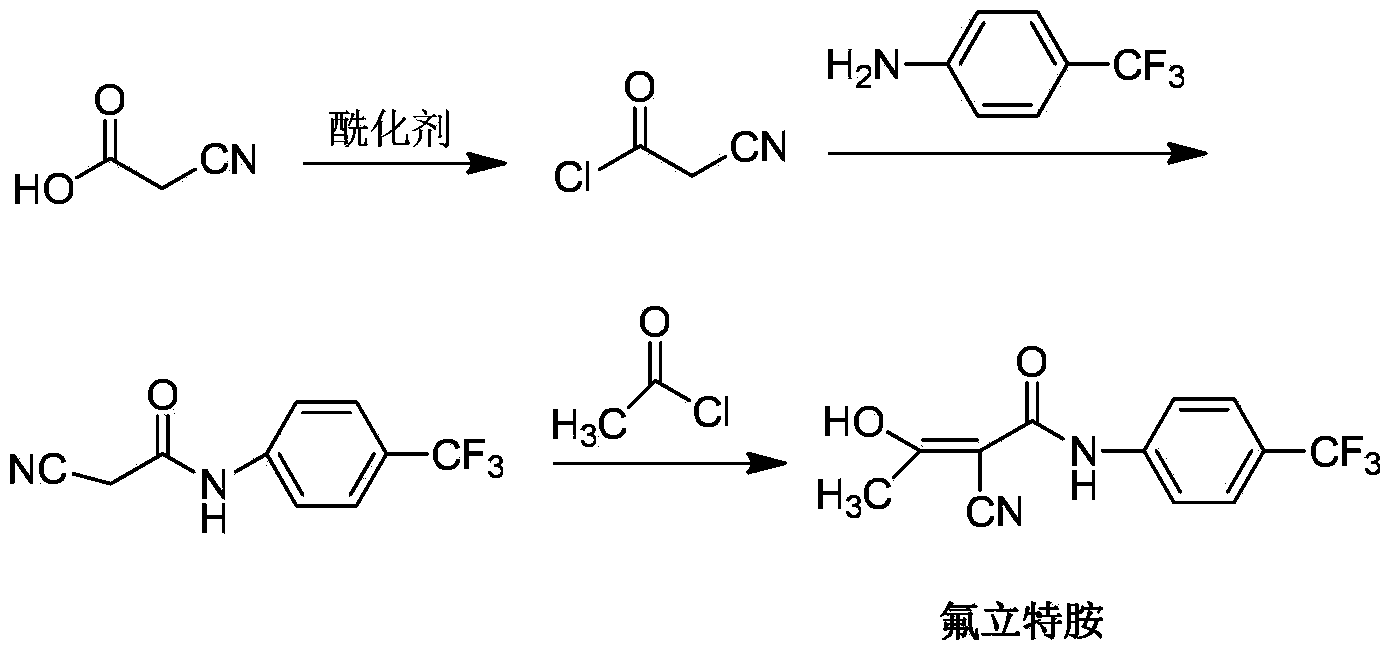
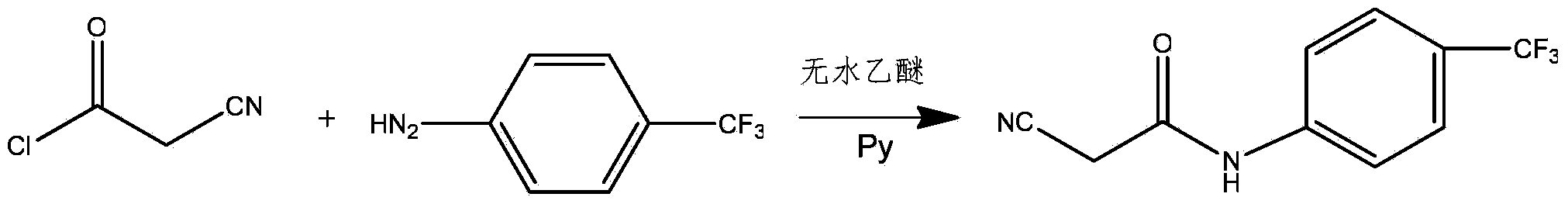
Preparation method of teriflunomide
InactiveCN103709068ARaw materials are easy to getMild reaction conditionsCarboxylic acid nitrile preparationOrganic compound preparationAcetyl chloridePara-trifluoromethylaniline
The invention relates to a preparation method of teriflunomide. The preparation method comprises the following steps: mixing cyano acetyl chloride, p-trifluoromethylaniline and an acid-binding agent according to the molar ratio of 1:(1-3):(8-11), and reacting to obtain 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide; mixing 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide, acetyl chloride and an acid-binding agent according to the molar ratio of 1:(1-5):(1-5) of reaction materials, and reacting to obtain teriflunomide. The process has the advantages that the raw materials are easily available, the reaction condition is mild and the yield is high, and the preparation method is suitable for industrial production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
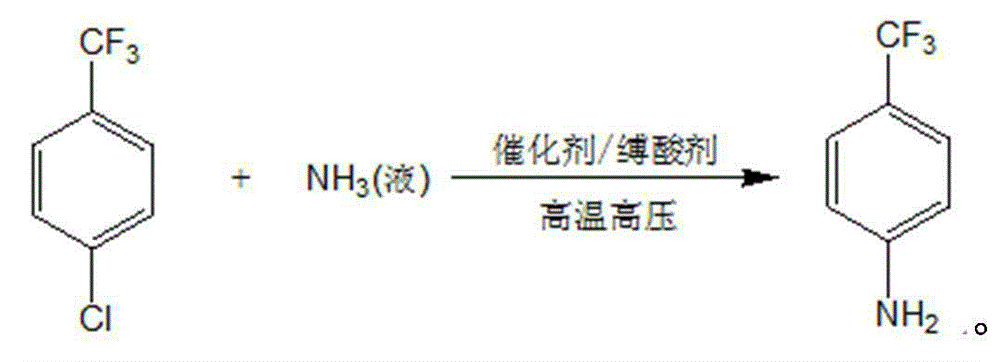
Method for preparing p-trifluoromethylaniline by performing high pressure ammonolysis
ActiveCN103408436AReduce lossHarm reductionOrganic compound preparationAmino compound preparationN dimethylformamideChlorobenzene
The invention relates to a new method for preparing p-trifluoromethylaniline by performing high pressure ammonolysis. P-trifluoromethyl chlorobenzene serving as a raw material is subjected to high-temperature high pressure ammonolysis reaction in a solvent under the action of a catalyst, liquid ammonia and an acid-binding agent to generate p-trifluoromethyl phenylamine; the catalyst is a mixture of cuprous chloride and copper powder; the acid-binding agent is one or two of inorganic base mixtures of sodium hydroxide and the like, or one or two of organic base mixtures of pyridine and triethylamine; and the solvent is one or two of mixing solvents of methanol, ethanol, polyethylene glycol 300-3,000 and N,N-dimethylformamide. The method has the advantages that the inorganic base, serving as the acid-binding agent, with low cost and easy availability is adopted, the loss of liquid ammonia in the reaction process is greatly reduced, and the production efficiency is improved; and the catalyst can be prepared from organic solvents with the cost as low as that of ethanol and less harm, so that the generation of hydrolysis side reaction is avoided, the purity of the product is high and the quality is high; and raw materials without being totally reacted can be recycled.
Owner:JIANGSU FENGHUA CHEM IND
Method for recycling wastes of trifluoromethyl phenylamine kettle residue
InactiveCN103880686AInhibit aggregationEasy to operateAmino compound purification/separationPara-trifluoromethylanilinePolymerization
The invention discloses a method for recycling wastes of trifluoromethyl phenylamine kettle residues. The method comprises the steps of 1) vacuum distillation: by taking the trifluoromethyl phenylamine kettle residues as raw materials, adding inorganic alkali according to the proportion of 2-3% of the mass of the trifluoromethyl phenylamine kettle residues, performing vacuum distillation to obtain a light-component mixture containing m-trifluoromethyl phenylamine and p-trifluoromethyl phenylamine; and 2) vacuum rectification: adding the inorganic alkali into the light-component mixture obtained in the step 1), and performing rectification to obtain m-trifluoromethyl phenylamine and p-trifluoromethyl phenylamine respectively. According to the method disclosed by the invention, a polymerization phenomenon during the process of distillation and rectification is effectively prevented by adopting the inorganic alkali; the method is simple in operation process and easy in industrial production; and by adopting the technical scheme of the method, the discharge of pollutants of the production process is reduced, and the comprehensive recycling of the solid wastes is realized.
Owner:JIANGSU FENGHUA CHEM IND
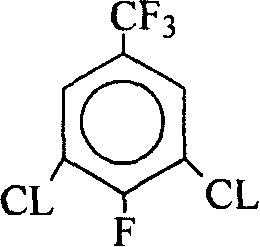
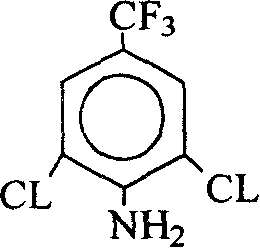
Method for producing 2,6-dichloro-4-trifluoromethylaniline
ActiveCN101165044ASimple and fast operationReduce manufacturing costOrganic compound preparationAmino compound preparationAnilineSolvent
The present invention discloses 2, 6-dichloro-p-trifluoro methyl aniline preparing process, which includes the first reaction of 3, 4, 5-trichloro trifluoro toluene and fluoride salt in solvent to produce 3, 5-dichloro-4-fluoro trifluoro toluene, and the subsequent reaction of 3, 5-dichloro-4-fluoro trifluoro toluene and liquid ammonia or ammonia water to obtain 2, 6-dichloro-p-trifluoro methyl aniline. The process has 3, 4, 5-trichloro trifluoro toluene as the side product from p-chloro trifluoro toluene producing process adopted, lowered production cost, no use of acid matter and less corrosion of the equipment.
Owner:SHANGHAI HETENG FINE CHEM
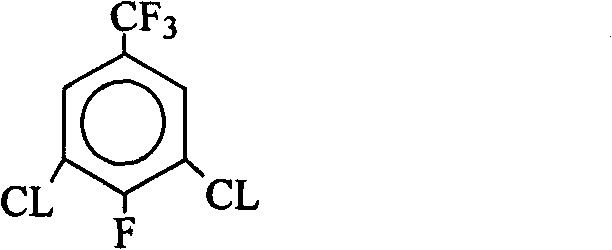
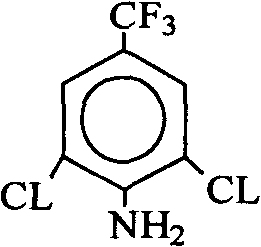
Preparation method of 1-[3-chloro-5-(trifluoromethyl) phenyl]-2, 2, 2-trifluoroethanone and derivatives thereof
ActiveCN112778109ASave raw materialsMild reaction conditionsOrganic compound preparationCarbonyl compound preparationCombinatorial chemistryPerylene derivatives
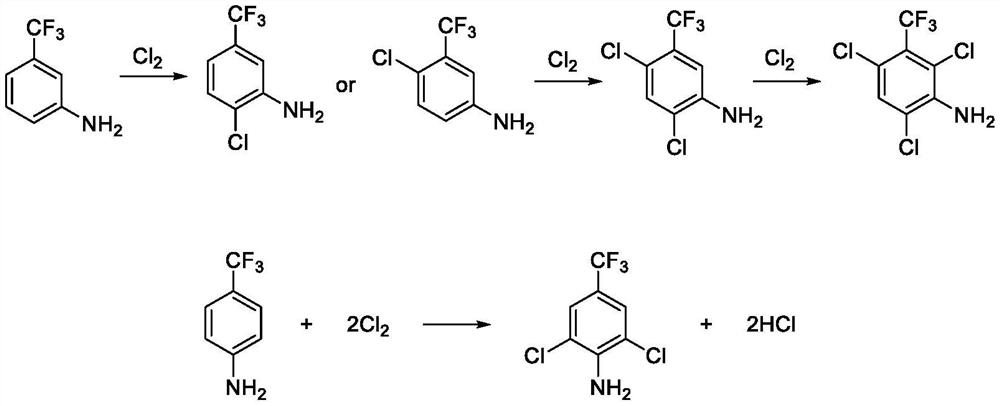
The invention relates to the field of synthesis of drug intermediates, in particular to a preparation method of 1-[3-chloro-5-(trifluoromethyl) phenyl]-2, 2, 2-trifluoroethanone and derivatives thereof, and the method comprises the following steps: synthesizing a compound II by p-trifluoromethylaniline, then synthesizing a compound III by chlorination, and finally synthesizing the 1-[3-chloro-5-(trifluoromethyl) phenyl]-2, 2, 2-trifluoroethanone and derivatives thereof by diazotization deamination. In the whole reaction, conditions are mild, raw materials are easy to obtain, economic effects of enterprises can be improved, production cost is reduced, and the method is suitable for large-scale production.
Owner:TAIZHOU ABSOBIOTEC CO LTD
Method for recycling wastes of trifluoromethyl phenylamine kettle residue
InactiveCN103880686BInhibit aggregationEasy to operateAmino compound purification/separationPara-trifluoromethylanilinePolymerization
Owner:JIANGSU FENGHUA CHEM IND
New synthesis method of 2,6-dichloro-4-trifluoromethylaniline
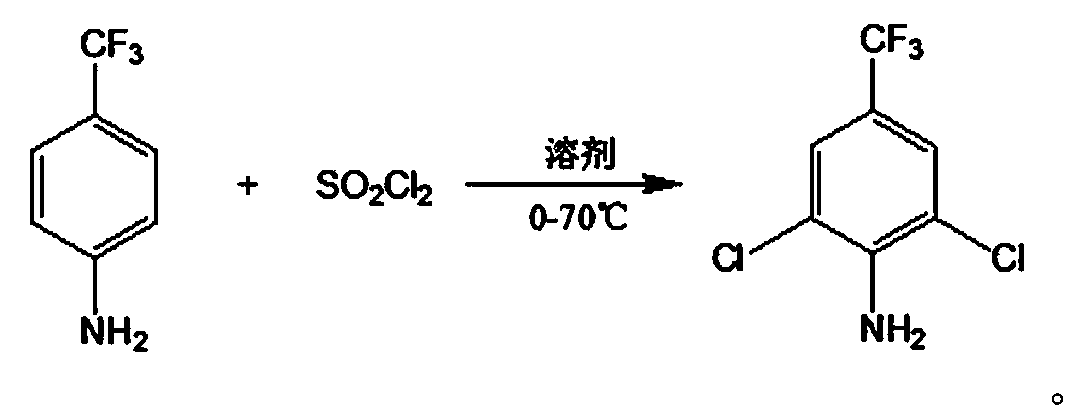
InactiveCN110655468ASimple processEasy to operateOrganic compound preparationAmino compound preparationSulfonyl chlorideMethylaniline
The invention discloses a new synthesis method of 2,6-dichloro-4-trifluoromethylaniline, wherein p-trifluoromethylaniline is usedas a raw material, and is added with a certain amount of sulfonyl chloride in a dropwise manner, and chlorination, water washing and pressure reducing rectification are performed to obtain the target compound. Compared with the method in the prior art, the method of theinvention has characteristics of simple process, easy operation, short reaction time, high yield and low production cost, is suitable for industrial production, is an environmentally-friendly chemicalprocess, and has good industrial application prospect.
Owner:JIANGSU FENGHUA CHEM IND
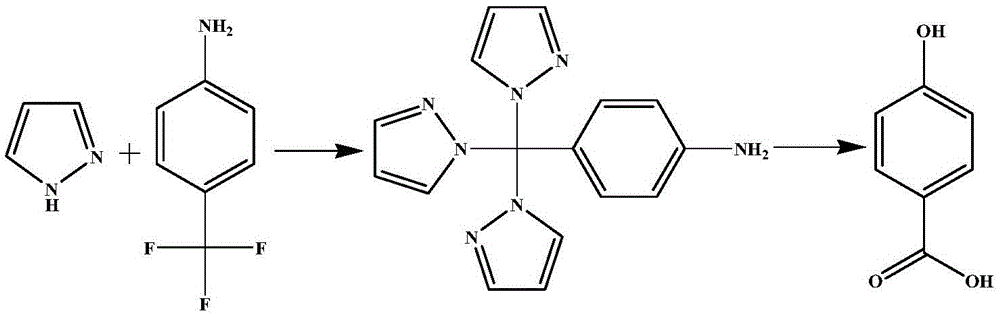
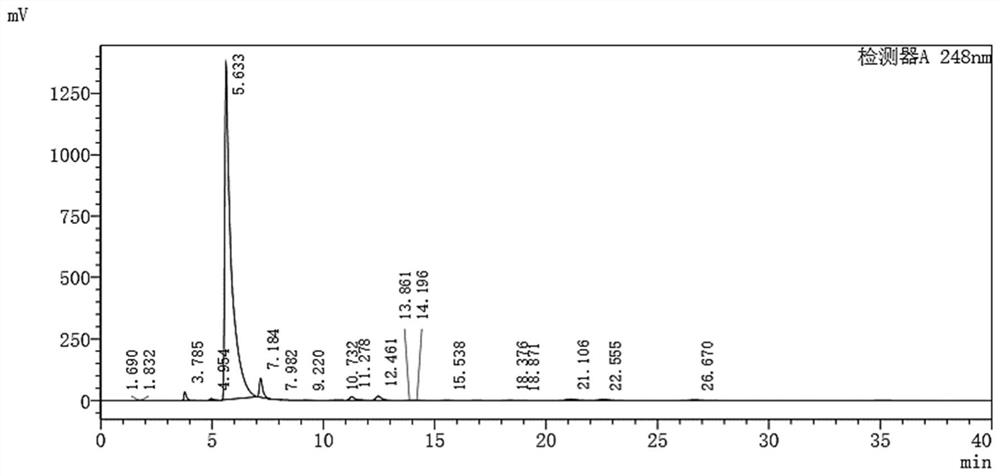
Preparation method for p-hydroxybenzoic acid
InactiveCN105481674AShort reaction timeHigh yieldOrganic compound preparationCarboxylic compound separation/purificationEvaporationPotassium hydroxide
The invention relates to a preparation method for p-hydroxybenzoic acid. The preparation method comprises successively adding pyrazole, 4-aminobenzotrifluoride and potassium hydroxide into dimethyl sulphoxide, dissolving, heating and refluxing for 1 h, cooling, adding water into the refluxing liquid for dissolving, adding dichloromethane, separating the liquid, extracting for three times, drying the organic phase with anhydrous magnesium sulfate, and performing column chromatography separation for obtaining 4-(tri(pyrazol-1-yl)methyl)aniline; and dropwise adding concentrated sulfuric acid into a mixed solution of 4-(tri(pyrazol-1-yl)methyl)aniline and water, dropwise adding a sodium nitrite aqueous solution under an ice bath condition with stirring, dropwise adding a sulfuric acid solution with the concentration of 15.5%, heating to 30-100 DEG C, reacting for 15 min to 2 h, naturally cooling, adding ethyl acetate and performing liquid-separation extraction for three times, performing reduced-pressure rotary evaporation, drying the organic phase with anhydrous sodium sulfate, and performing vacuum drying, so as to obtain p-hydroxybenzoic acid. The reaction synthetic route is short, reaction time is short, yield is high, and postprocessing is simple. The yield of 4-(tri(pyrazol-1-yl)methyl)aniline is about 80%, and the yield of p-hydroxybenzoic acid is about 99%.
Owner:HUBEI UNIV OF TECH
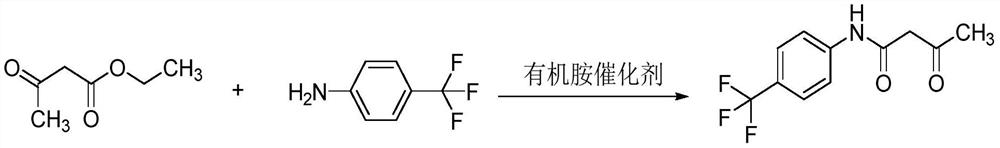
Preparation method of 3-oxo-N-(4-trifluoromethylphenyl) butylamide
PendingCN112457210AHigh yieldHigh purityOrganic compound preparationCarboxylic acid amides preparationPtru catalystOrganic base
The invention relates to a preparation method of 3-oxo-N-(4-trifluoromethylphenyl) butylamide, wherein the preparation method comprises the steps: carrying out amidation reaction on ethyl acetoacetateand p-trifluoromethylaniline to obtain 3-oxo-N-(4-trifluoromethylphenyl) butylamide, wherein in the reaction process, excessive p-trifluoromethylaniline is used as a solvent, and a catalyst adopted in the reaction process is an organic base catalyst. Compared with the prior art, the preparation method has the advantages that ethyl acetoacetate and p-trifluoromethylaniline are subjected to amidation reaction, and the product 3-oxo-N-(4-trifluoromethylphenyl) butylamide is obtained through post-treatment, wherein the synthesis operation of the 3-oxo-N-(4-trifluoromethylphenyl) butylamide is simple, the cost is low, the yield is high, the use of volatile toxic organic solvents and the emission of waste gas are avoided, and the process route is green and environment-friendly; and the obtainedproduct has few impurities, the target product has high purity, the raw materials are easy to recycle, the post-treatment operation is simple, the reaction time is shortened, and the method is suitable for industrial production.
Owner:SHANGHAI INST OF TECH +1
The preparation method of flutermide
InactiveCN103709068BRaw materials are easy to getMild reaction conditionsCarboxylic acid nitrile preparationOrganic compound preparationAcetyl chloridePhenyl group
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Method for preparing carbonyl fluoride from p-trichloromethylphenyl isocyanate
ActiveCN107473198AHigh reaction yieldHigh purityChlorine/hydrogen-chloride purificationCarbon compoundsHydrogen fluorideCarbonyl fluoride
The invention provides a method for preparing carbonyl fluoride from p-trichloromethylphenyl isocyanate. The method is a new one in which the carbonyl fluoride is safely and effectively obtained by reacting hydrogen fluoride and phenyl isocyanate; and the method can ensure that the reaction proceeds towards the direction of generating the carbonyl fluoride maximally. The method provided by the invention comprises the following steps: in the presence of the phenyl isocyanate and the excessive hydrogen fluoride, performing a first reaction on the hydrogen fluoride and the phenyl isocyanate at a low temperature; performing heating to perform a second reaction; collecting a p-trifluoromethyl aniline product; and performing cooling to collect the liquid hydrogen fluoride, introducing the uncondensed gas into a rectification tower, performing rectification, and performing separation to obtain hydrogen chloride and the carbonyl fluoride. The method provided by the invention can ensure high reaction yield, high product purity, mild reaction conditions and favorable safety; and on the basis of producing the carbonyl fluoride, the hydrogen fluoride, the hydrogen chloride and the p-trifluoromethyl aniline can be produced simultaneously, and no pollutant is discharged, thereby meeting environmental requirements.
Owner:ZIBO FEIYUAN CHEM CO LTD
Continuous flow teriflunomide preparation process
InactiveCN113666842ASolve the impact of damageAdequate responseCarboxylic acid nitrile preparationOrganic compound preparationAcetyl chlorideBiochemical engineering
The invention discloses a continuous flow teriflunomide preparation process which comprises the following steps: using a continuous flow reactor, taking cyanoacetic acid as a starting material, preparing cyanoacetyl chloride through chlorination, synthesizing a teriflunomide intermediate through the cyanoacetyl chloride and p-trifluoromethylaniline, and synthesizing teriflunomide through the intermediate and acetyl chloride. The method has the advantages of high safety, low cost, low energy consumption and high production yield.
Owner:河北凯威恒诚制药有限公司
Synthetic method of p-trifluoromethylaniline
InactiveCN101298421BLow priceNo emissionsPhysical/chemical process catalystsOrganic compound preparationP-chlorobenzotrifluorideOrganic solvent
Owner:太仓中化环保化工有限公司
Method for preparing 2, 6-dichloro-4-trifluoromethylaniline
PendingCN114507147ASimple processShort reaction timeAmino compound purification/separationOrganic compound preparationMethylanilineCombinatorial chemistry
The invention discloses a method for preparing 2, 6-dichloro-4-(trifluoromethyl) aniline and a preparation method of the 2, 6-dichloro-4-(trifluoromethyl) aniline. The method for preparing 2, 6-dichloro-4-trifluoromethylaniline comprises the following steps: enabling m-trifluoromethylaniline residues in a rectifying still to react with chlorine in a solvent until the content of 2, 4-dichloro-5-trifluoromethylaniline in reaction liquid is less than or equal to 0.3%, thereby obtaining the 2, 6-dichloro-4-trifluoromethylaniline, the rectifying still residue of the m-trifluoromethylaniline is prepared from the following components: 69.6 percent to 72.5 percent of p-trifluoromethylaniline and 25.5 percent to 28.4 percent of the m-trifluoromethylaniline; the solvent is water or hydrochloric acid. The process is simple, melt crystallization is not needed, the purity of the prepared 2, 6-dichloro-4-trifluoromethylaniline product is up to 99.5%, and the percentage is mass percentage.
Owner:ZHEJIANG WEIHUA NEW MATERIAL CO LTD
Production process of p-trifluoromethyl aniline
ActiveCN100349853COrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPotassium fluorideAniline
The present invention discloses the production process of p-trifluoromethyl aniline. P-trifluorotoluene and liquid ammonia are reacted in the presence of alcohol and catalyst to obtain p-trifluoromethyl aniline. The catalyst may consist of ferrocene, triphenyl phosphor, potassium fluoride, ammonium tetrabutyl bromide. The present invention has high efficient composite catalyst used, single pass conversion rate up to 66 % and yield up to 90 %. The organic solvent may be used circularly, the excessive liquid ammonia may be prepared into ammonia water, the catalyst may be regenerated and reused, and the process has no waste water exhausted.
Owner:江苏优普生物化学科技股份有限公司
Catalyst residue reduction and resource utilization method
PendingCN112676307ADoes not affect the catalytic effectReduce solid wasteSolid waste disposalChlorobenzenePtru catalyst
The invention discloses a catalyst residue reduction and resource utilization method. The method comprises the following steps that firstly, pressure-reduced drying is carried out on filter cakes obtained by pressure filtration after desolventizing of p-trifluoromethylaniline synthetic liquid, and p-trifluoromethylaniline and p-trifluoromethyl chlorobenzene are recycled by dried condensate through rectification; and secondly, the dried filter cakes are crushed and are heated to 280-350 DEG C, and the filter cakes are condensed and crystallized again to be separated out after ammonium chloride is decomposed. According to the scheme, p-trifluoromethylaniline catalyst residues are dried through a conventional industrial method, main components in the dried condensate are p-trifluoromethyl chlorobenzene and p-trifluoromethylaniline, the dried condensate can be merged into a rectification raw material of a p-trifluoromethylaniline production system, p-trifluoromethylaniline and p-trifluoromethyl chlorobenzene are separated through rectification, p-trifluoromethylaniline serves as a finished product, and p-trifluoromethyl chlorobenzene serves as the raw material. After treatment of the scheme, a small amount of acidic wastewater is generated, and solid waste can be reduced by more than 65%.
Owner:江苏优普生物化学科技股份有限公司
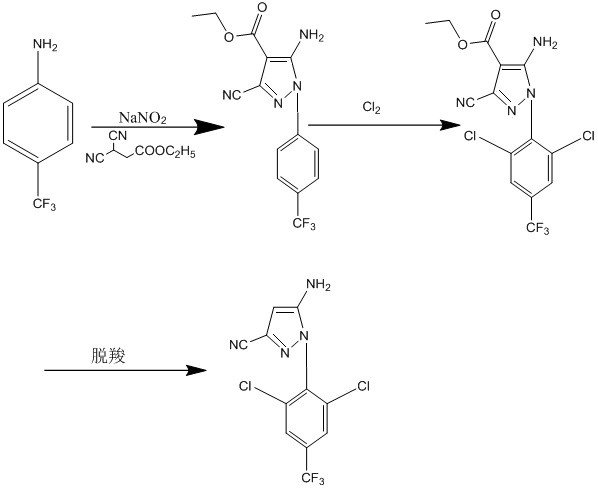
Preparation method of 5-amino-3-cyano-1-(2, 6-dichloro-4-trifluoromethylphenyl)pyrazole
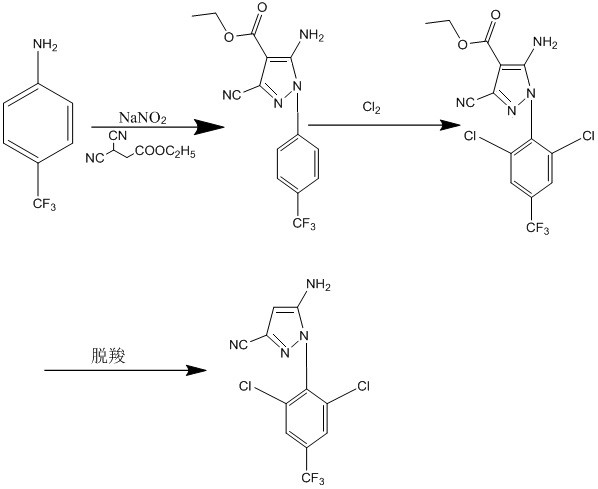
ActiveCN112094235ASimple and safe operationGood choiceOrganic chemistryPropanoic acidEthyl propiolate
The invention provides a preparation method of 5-amino-3-cyano-1-(2, 6-dichloro-4-trifluoromethylphenyl)pyrazole. The preparation method comprises the following steps of by taking p-trifluoromethylaniline as a raw material, carrying out diazotization reaction to form a ring with ethyl 2, 3-dicyanopropionate under a weakly acidic condition, and introducing a proper amount of chlorine into the cyclization compound for chlorination, and adjusting the pH value for decarboxylation to obtain the product 5-amino-3-cyano-1-(2, 6-dichloro-4-trifluoromethylphenyl)pyrazole. The preparation method of the5-amino-3-cyano-1-(2, 6-dichloro-4-trifluoromethyl phenyl)pyrazole is simple to operate, good in chlorination selectivity, few in side reaction, high in product yield, mild in reaction condition and beneficial to industrialization; the average yield of the method is not lower than 95%, and the product purity is 99.50% or above.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Method for producing 2,6-dichloro-4-trifluoromethylaniline
ActiveCN101165044BSimple and fast operationReduce manufacturing costOrganic compound preparationAmino compound preparationSolventToluene
The present invention discloses 2, 6-dichloro-p-trifluoro methyl aniline preparing process, which includes the first reaction of 3, 4, 5-trichloro trifluoro toluene and fluoride salt in solvent to produce 3, 5-dichloro-4-fluoro trifluoro toluene, and the subsequent reaction of 3, 5-dichloro-4-fluoro trifluoro toluene and liquid ammonia or ammonia water to obtain 2, 6-dichloro-p-trifluoro methyl aniline. The process has 3, 4, 5-trichloro trifluoro toluene as the side product from p-chloro trifluoro toluene producing process adopted, lowered production cost, no use of acid matter and less corrosion of the equipment.
Owner:SHANGHAI HETENG FINE CHEM
A kind of preparation method of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole
ActiveCN112094235BAvoid harmHigh safety in industrial productionOrganic chemistryPropanoic acidWater chlorination
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Synthesis method of triphenyl-4-(trifluoromethylbenzamide)butylphosphonium chloride and application of triphenyl-4-(trifluoromethylbenzamide)butylphosphonium chloride in antitumor drugs
InactiveCN109096332AOvercoming harsh, difficult to control,Overcome purityGroup 5/15 element organic compoundsAntineoplastic agentsPhosphonium saltSynthesis methods
The invention relates to a synthesis method of triphenyl-4-(trifluoromethylbenzamide)butylphosphonium chloride and an application of triphenyl-4-(trifluoromethylbenzamide)butylphosphonium chloride inantitumor drugs, and belongs to the technical field of organic synthesis. The method comprises the following steps: taking o-trifluoromethylaniline, m-trifluoromethylaniline and p-trifluoromethylaniline as raw materials, chloroform as a solvent and sodium carbonate, triethylamine, pyridine and the like as an acid binding agent to be subjected to reaction with 4-chlorobutyryl chloride so as to obtain a first-grade product, and performing reflux on the obtained first-grade product and triphenylphosphine in the solvent to obtain the final product trifluoromethylbenzamide triphenyl quaternary phosphonium salt. The target product is not limited by the types of the acid binding agents and solvents, and defects of harsh synthesis conditions, uneasy control and low purity of the synthesized product in the prior art are overcome; besides, the step-by-step elution procedure is introduced into purification of the final product, the selected specific eluent ensures purity of the final product. Theraw materials are widely sourced, cheap and easily available, and the prepared quaternary phosphonium salt compound is low in toxicity and efficient, and triphenyl-4-(trifluoromethylbenzamide)butylphosphonium chloride can be applied to preparation of the antitumor drugs.
Owner:HUBEI UNIV
A kind of method utilizing p-trichloromethylbenzene isocyanate to manufacture carbonyl fluoride
ActiveCN107473198BHigh reaction yieldHigh purityChlorine/hydrogen-chloride purificationCarbon compoundsHydrogen fluorideCarbonyl fluoride
The invention provides a method for preparing carbonyl fluoride from p-trichloromethylphenyl isocyanate. The method is a new one in which the carbonyl fluoride is safely and effectively obtained by reacting hydrogen fluoride and phenyl isocyanate; and the method can ensure that the reaction proceeds towards the direction of generating the carbonyl fluoride maximally. The method provided by the invention comprises the following steps: in the presence of the phenyl isocyanate and the excessive hydrogen fluoride, performing a first reaction on the hydrogen fluoride and the phenyl isocyanate at a low temperature; performing heating to perform a second reaction; collecting a p-trifluoromethyl aniline product; and performing cooling to collect the liquid hydrogen fluoride, introducing the uncondensed gas into a rectification tower, performing rectification, and performing separation to obtain hydrogen chloride and the carbonyl fluoride. The method provided by the invention can ensure high reaction yield, high product purity, mild reaction conditions and favorable safety; and on the basis of producing the carbonyl fluoride, the hydrogen fluoride, the hydrogen chloride and the p-trifluoromethyl aniline can be produced simultaneously, and no pollutant is discharged, thereby meeting environmental requirements.
Owner:ZIBO FEIYUAN CHEM CO LTD
Method for preparing p-trifluoromethylaniline by performing high pressure ammonolysis
ActiveCN103408436BReduce lossHarm reductionOrganic compound preparationAmino compound preparationN dimethylformamideChlorobenzene
The invention relates to a new method for preparing p-trifluoromethylaniline by performing high pressure ammonolysis. P-trifluoromethyl chlorobenzene serving as a raw material is subjected to high-temperature high pressure ammonolysis reaction in a solvent under the action of a catalyst, liquid ammonia and an acid-binding agent to generate p-trifluoromethyl phenylamine; the catalyst is a mixture of cuprous chloride and copper powder; the acid-binding agent is one or two of inorganic base mixtures of sodium hydroxide and the like, or one or two of organic base mixtures of pyridine and triethylamine; and the solvent is one or two of mixing solvents of methanol, ethanol, polyethylene glycol 300-3,000 and N,N-dimethylformamide. The method has the advantages that the inorganic base, serving as the acid-binding agent, with low cost and easy availability is adopted, the loss of liquid ammonia in the reaction process is greatly reduced, and the production efficiency is improved; and the catalyst can be prepared from organic solvents with the cost as low as that of ethanol and less harm, so that the generation of hydrolysis side reaction is avoided, the purity of the product is high and the quality is high; and raw materials without being totally reacted can be recycled.
Owner:JIANGSU FENGHUA CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


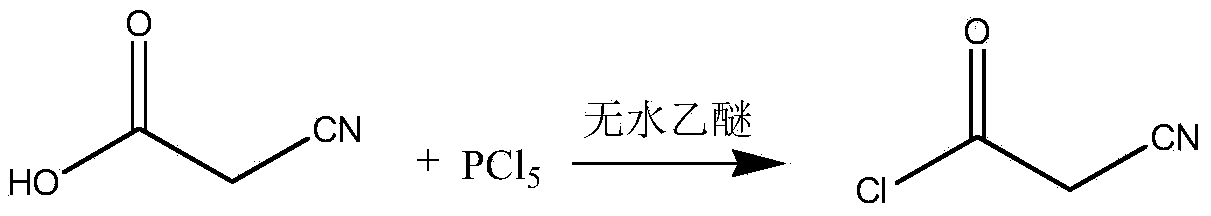
![Preparation method of 1-[3-chloro-5-(trifluoromethyl) phenyl]-2, 2, 2-trifluoroethanone and derivatives thereof Preparation method of 1-[3-chloro-5-(trifluoromethyl) phenyl]-2, 2, 2-trifluoroethanone and derivatives thereof](https://images-eureka.patsnap.com/patent_img/00b72d6a-89dd-44ad-8e45-e9e337d74707/DEST_PATH_IMAGE001.png)
![Preparation method of 1-[3-chloro-5-(trifluoromethyl) phenyl]-2, 2, 2-trifluoroethanone and derivatives thereof Preparation method of 1-[3-chloro-5-(trifluoromethyl) phenyl]-2, 2, 2-trifluoroethanone and derivatives thereof](https://images-eureka.patsnap.com/patent_img/00b72d6a-89dd-44ad-8e45-e9e337d74707/DEST_PATH_IMAGE003.png)
![Preparation method of 1-[3-chloro-5-(trifluoromethyl) phenyl]-2, 2, 2-trifluoroethanone and derivatives thereof Preparation method of 1-[3-chloro-5-(trifluoromethyl) phenyl]-2, 2, 2-trifluoroethanone and derivatives thereof](https://images-eureka.patsnap.com/patent_img/00b72d6a-89dd-44ad-8e45-e9e337d74707/347164DEST_PATH_IMAGE002.png)