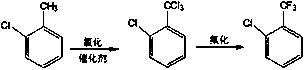
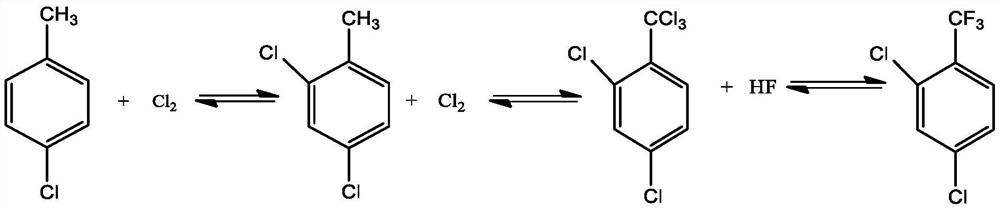
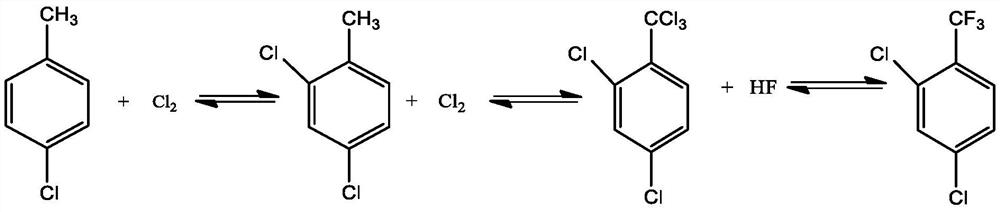
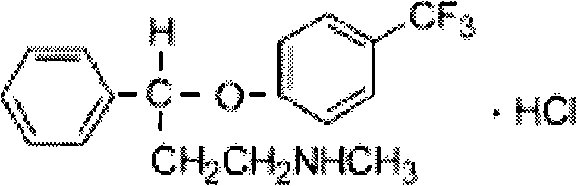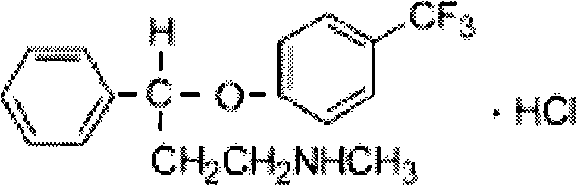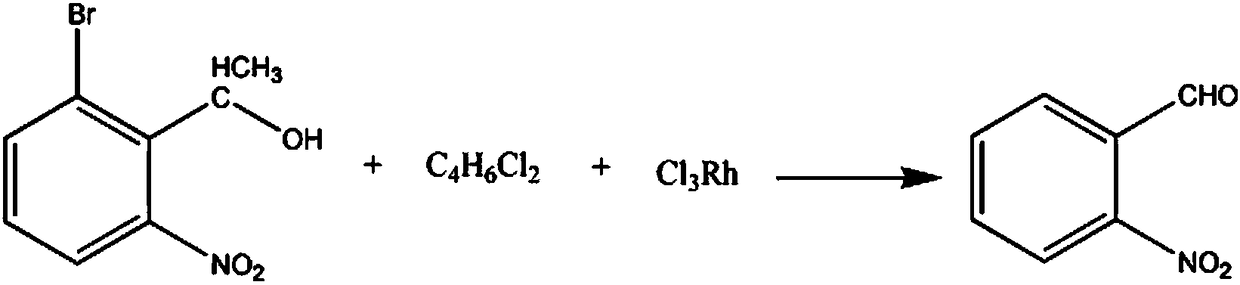Patents
Literature
32 results about "P-chlorobenzotrifluoride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
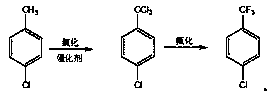
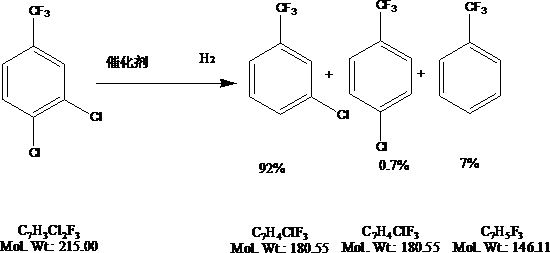
Parachlorobenzotrifluoride is an organic chemical compound with the molecular formula C7H4ClF3. It is frequently abbreviated PCBTF. Parachlorobenzotrifluoride is a colorless liquid with a distinct aromatic odor.
Production method of p-trifluoromethylaniline
InactiveCN102643202APhysical/chemical process catalystsOrganic compound preparationP-chlorobenzotrifluorideAlcohol
The invention discloses a production method of p-trifluoromethylaniline. The production method comprises the following step: enabling p-chlorobenzotrifluoride and liquid ammonia to react in the presence of alcohol and a catalyst to get the p-trifluoromethylaniline, wherein the catalyst mainly comprises cuprous chloride and potassium fluoride. According to the production method disclosed by the invention, the high-efficient compound catalyst is adopted, and the yield can achieve 90%; and an organic solvent in the production method can be applied mechanically in a circulating manner, no wastewater exists, the excess liquid ammonia can be produced into ammonia water for selling, and the catalyst can be recycled.
Owner:江苏优普生物化学科技股份有限公司
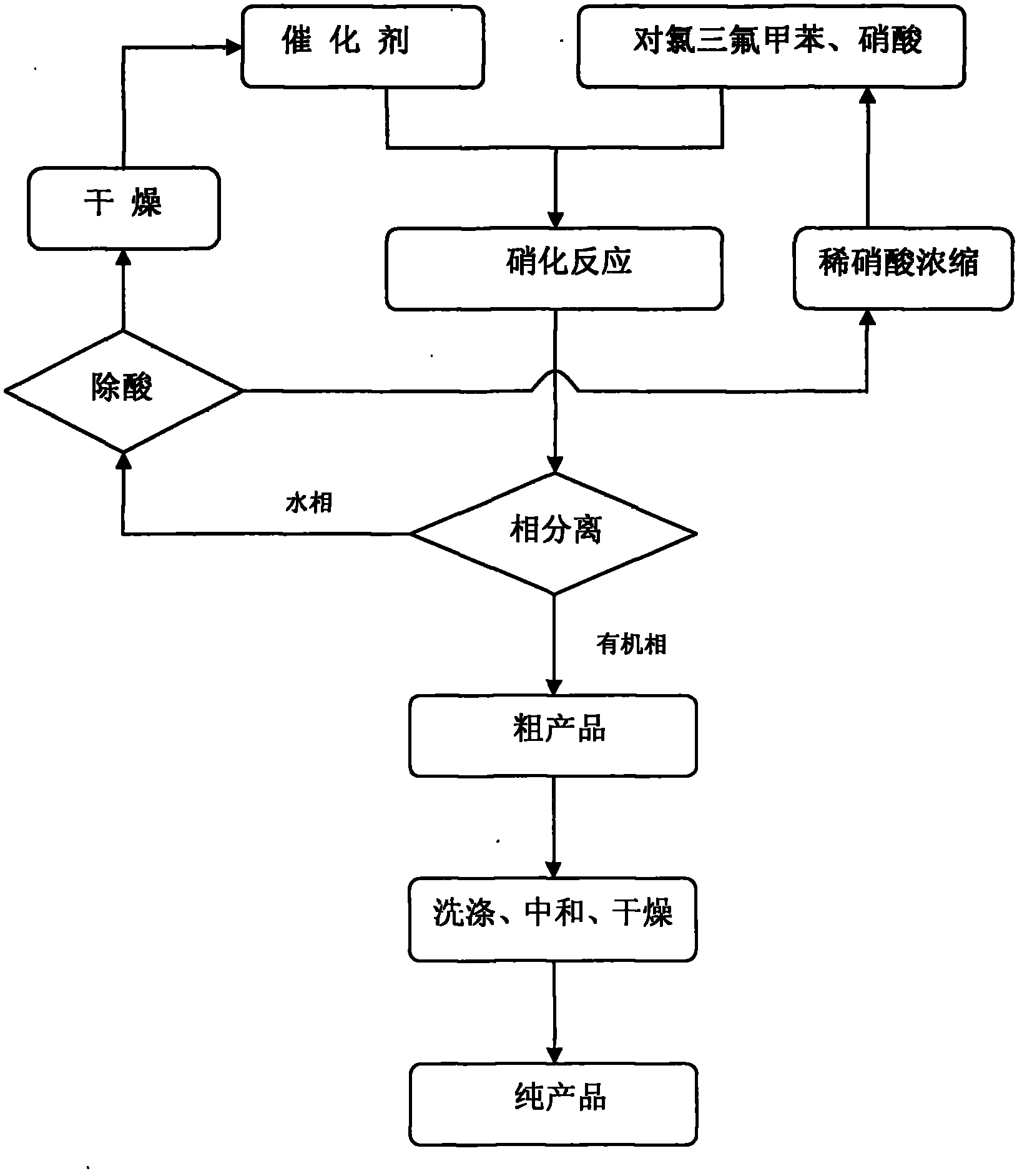
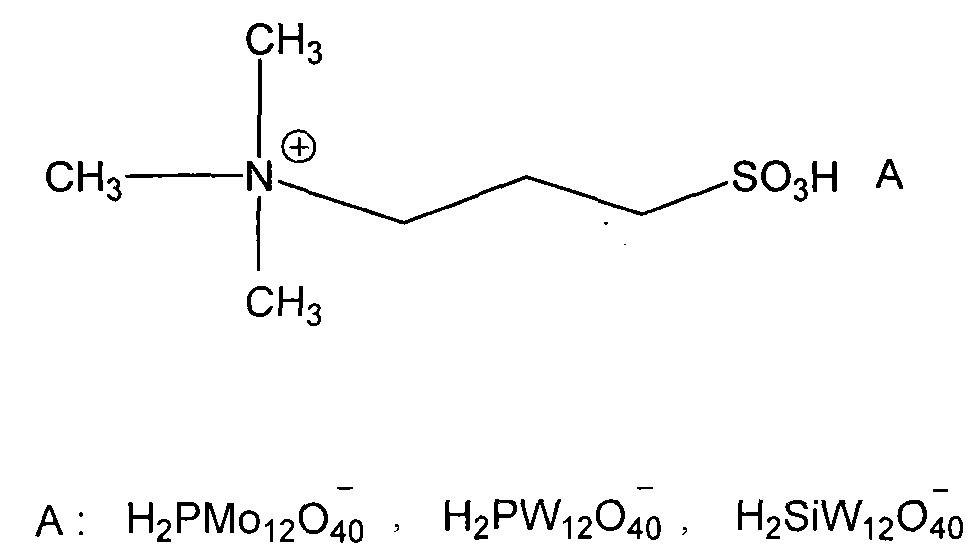
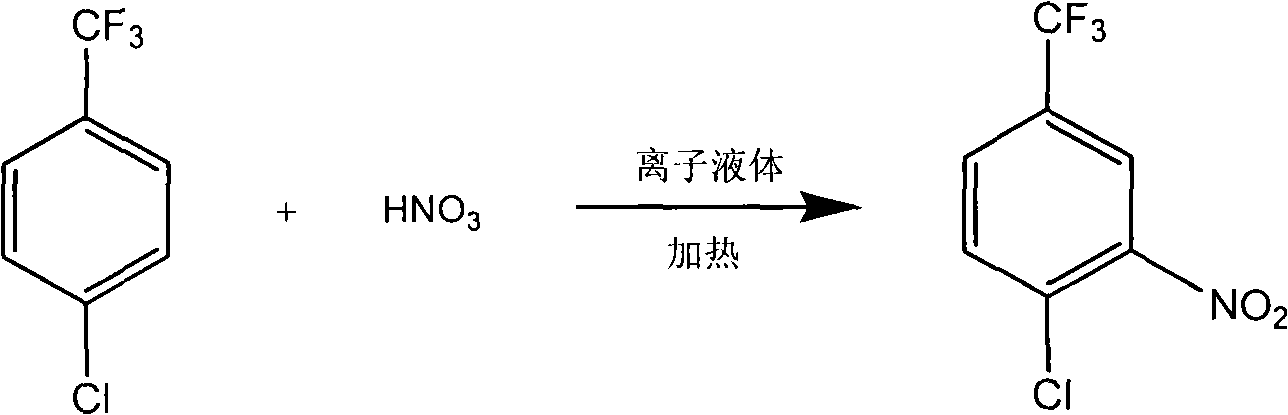
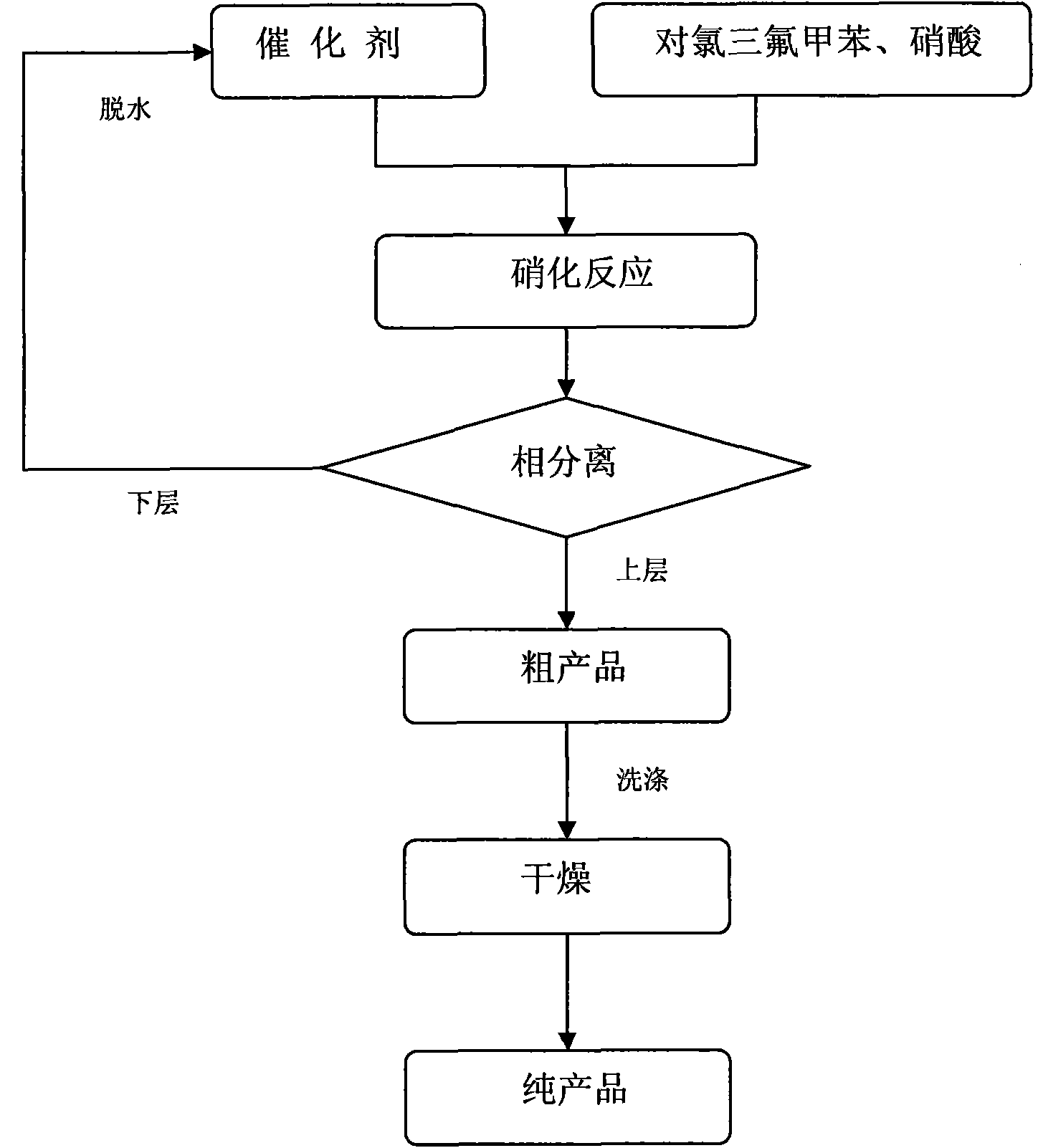
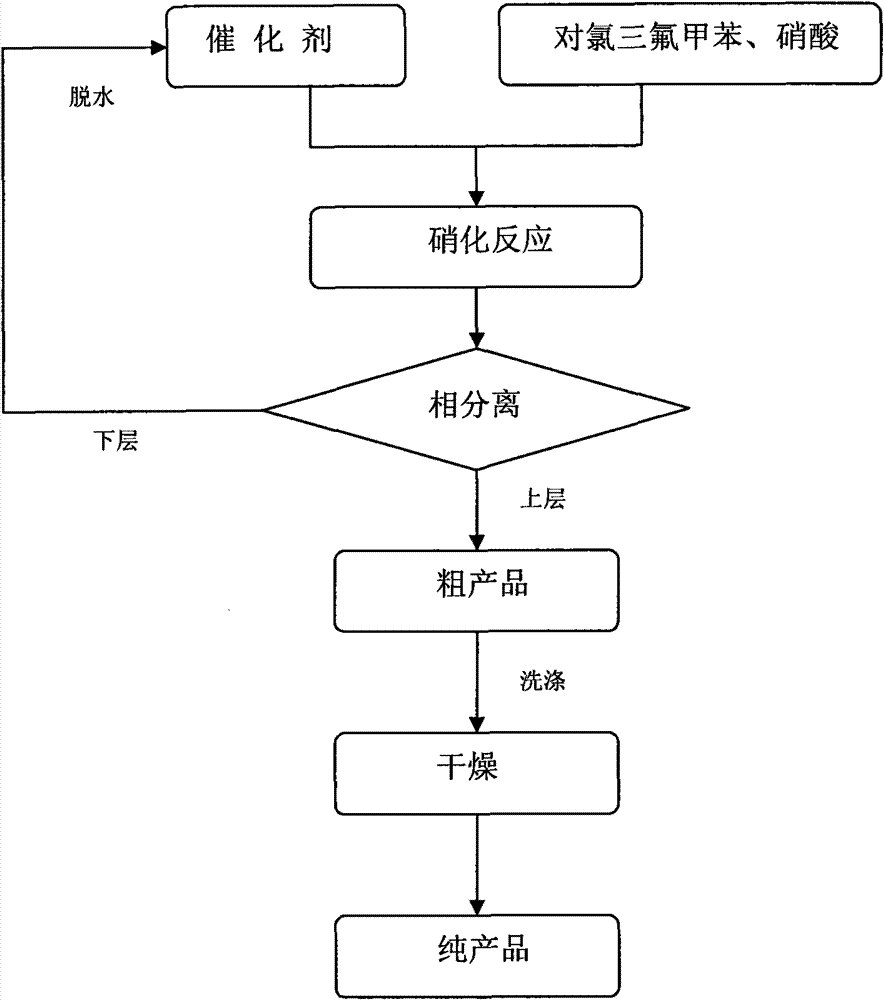
P-chlorobenzotrifluoride clean nitration reaction catalyzed by heteropoly acid ionic liquid
InactiveCN102417457AReduce dosageWide variety of sourcesOrganic-compounds/hydrides/coordination-complexes catalystsNitro compound preparationP-chlorobenzotrifluorideHeteropoly acid
The invention discloses a novel method for carrying out p-chlorobenzotrifluoride clean nitration reaction catalyzed by a heteropoly acid ionic liquid. The used catalyst is an ionic liquid having a heteropoly acid anionic structure, and p-chlorobenzotrifluoride and nitric acid used as raw materials are subjected to nitration reaction under the action of the catalyst, thus obtaining 4-chloro-3-nitrobenzotrifluoride. Compared with the prior art, the invention has the following advantages: (1) the used ionic liquid having a heteropoly acid anionic structure has a wide source of raw materials and is convenient to prepare; and the catalyst is stable in water, can not be inactivated and can be used circularly; (2) compared with corresponding inorganic acid, the heteropoly acid forming the anionic structure has higher strength, higher catalytic activity, less consumption and better environment friendliness; and (3) by using the ionic liquid instead of concentrated sulfuric acid, the nitration process is environment-friendly and has industrial application prospects.
Owner:YANCHENG TEACHERS UNIV
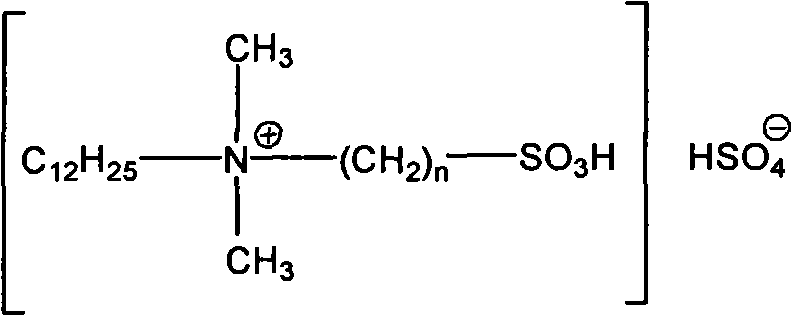
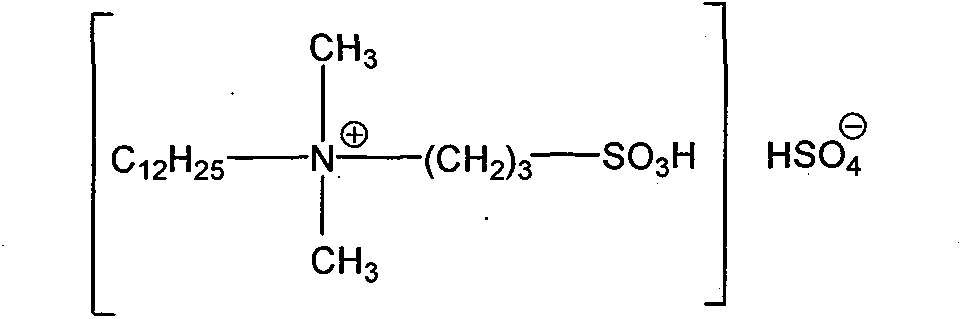
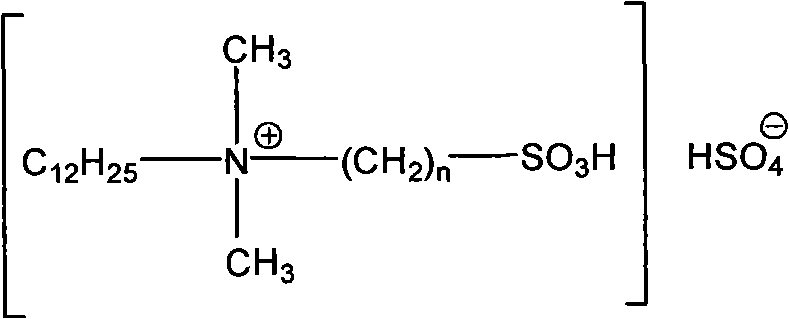
Clean nitration reaction of p-chlorobenzotrifluoride under catalysis of degradable functional ionic liquid
ActiveCN102249927AWide variety of sourcesEasy to manufactureOrganic-compounds/hydrides/coordination-complexes catalystsNitro compound preparationQuaternary ammonium cationP-chlorobenzotrifluoride
The invention discloses a new method of clean nitration reaction of p-chlorobenzotrifluoride under catalysis of degradable functional ionic liquid. Biodegradable ionic liquid of quaternary ammonium cation structure is used as a catalyst, p-chlorobenzotrifluoride and nitric acid are used as raw materials, and 4-chloro-3-nitrobenzotrifluoride is obtained by nitration reaction under the action of the catalyst. Compared with the prior art, the method has the advantages that: (1) because the degradable ionic liquid of quaternary ammonium cation structure is adopted, the raw material source is extensive, and the preparation is convenient; the catalyst has high activity, low consumption and water stability, is not inactivated and can be recycled; (2) the ionic liquid is biodegradable and environment-friendly; and (3) because the ionic liquid is used for substituting concentrated sulfuric acid, the method is an environment-friendly chemical process, and has good industrialized application prospect.
Owner:JIANGSU DAHUA CHEM IND
Synthetic method of p-trifluoromethylaniline
InactiveCN101298421ALow priceNo emissionsPhysical/chemical process catalystsOrganic compound preparationOrganic solventP-chlorobenzotrifluoride
The invention relates to a synthetic method of p-trifluoromethylaniline; p-chlorobenzotrifluoride and liquid ammonia generate an ammonolysis reaction to generate the p-trifluoromethylaniline under the action of a catalyst and an acid-binding agent; according to portion by weight, the components of the catalyst is: cuprous chloride of 1 to 10 portions, potassium fluoride of 2 to 30 portions and phase transfer catalyst of 5 to 30 portions; the adding amount of the catalyst is 8 to 70 percent of the p-chlorobenzotrifluoride (mass); the synthetic method of the invention adopts an effective compound catalyst to provide a synthetic scheme which has higher one way conversion rate and is economically practical in industry for the synthesis of the p-trifluoromethylaniline; the price of the catalyst used in the synthetic process is lower; furthermore, an organic solvent and the phase transfer catalyst can be recycled and reused; the manufacture cost is low; no waste water is discharged during the whole technique process; and the synthetic method of the invention is friendly to the environment.
Owner:太仓中化环保化工有限公司
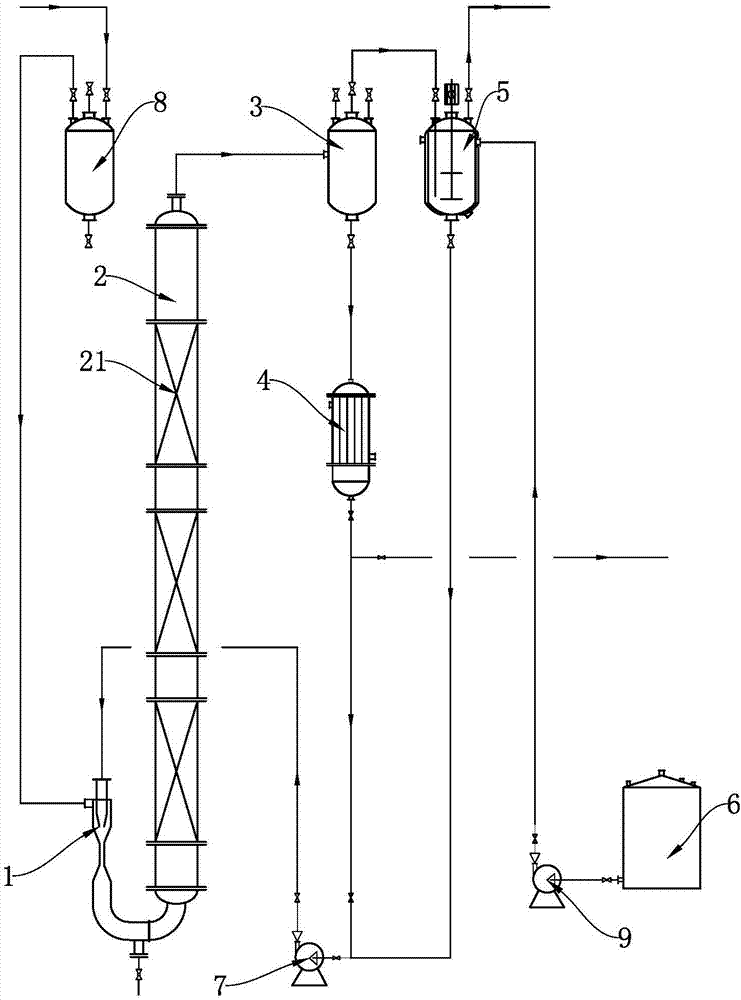
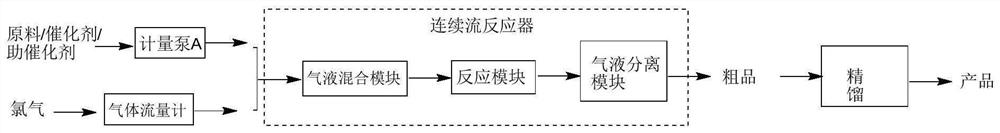
Continuous synthetic method and synthetic equipment for 3,4-dichlorotrifluoromethyl benzene
ActiveCN106892792AOvercoming intractable problemsContinuous productionEnergy inputHalogenated hydrocarbon preparationBenzeneP-chlorobenzotrifluoride
The invention discloses a continuous synthetic method and synthetic equipment for 3,4-dichlorotrifluoromethyl benzene. The continuous synthetic method comprises the following steps: using chlorine and p-chlorobenzotrifluoride as raw materials; sufficiently mixing the chlorine and the p-chlorobenzotrifluoride as the raw materials with a circulating material by a raw material mixer; then enabling a mixture to enter a chlorination tower filled with iron filler from the bottom, and carrying out continuous chlorination reaction; using one part of a reactant obtained from the top of the chlorination tower as the circulating material, and enabling the circulating material to return to the raw material mixer for carrying out continuous mixed reaction; refining residual materials to obtain a product 3,4-dichlorotrifluoromethyl benzene. The synthetic equipment comprises a feeding mixer, wherein an outlet of the feeding mixer is communicated with a lower inlet of the chlorination tower; a gas-liquid separator is communicated with a top outlet of the chlorination tower; a liquid phase outlet of the gas-liquid separator is respectively communicated with a product refining device and a liquid phase raw material inlet of the feeding mixer; the iron filler fills the chlorination tower. The continuous synthetic method and the synthetic equipment disclosed by the invention disclosed by the invention can realize continuous production and have the advantages of high capacity, low energy consumption, simplicity and convenience in operation and environment friendliness.
Owner:SHANDONG DOCRIS CHEM
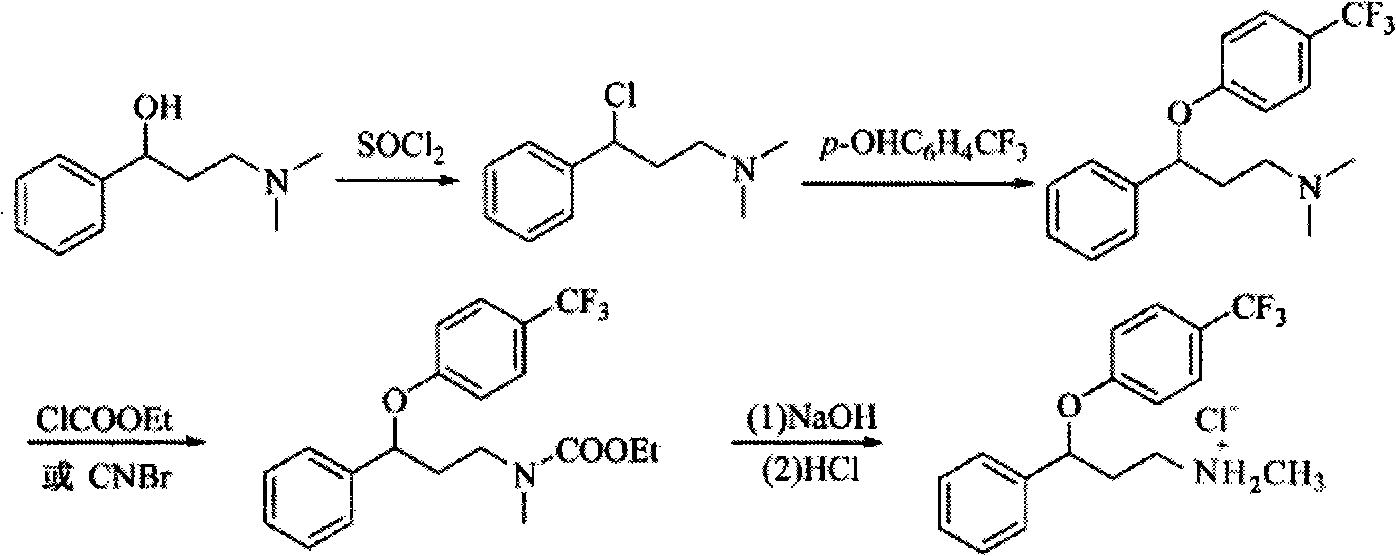
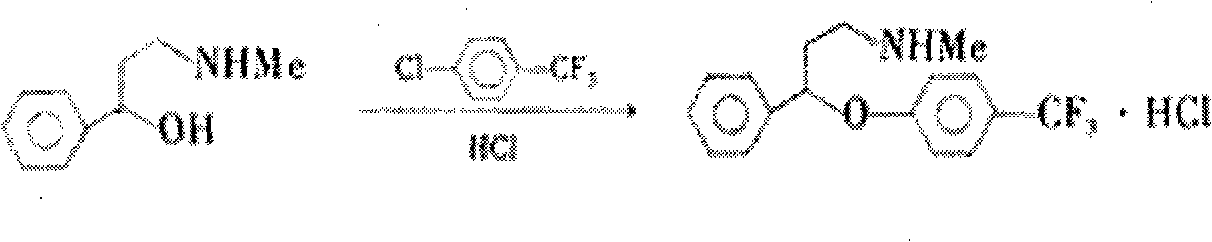
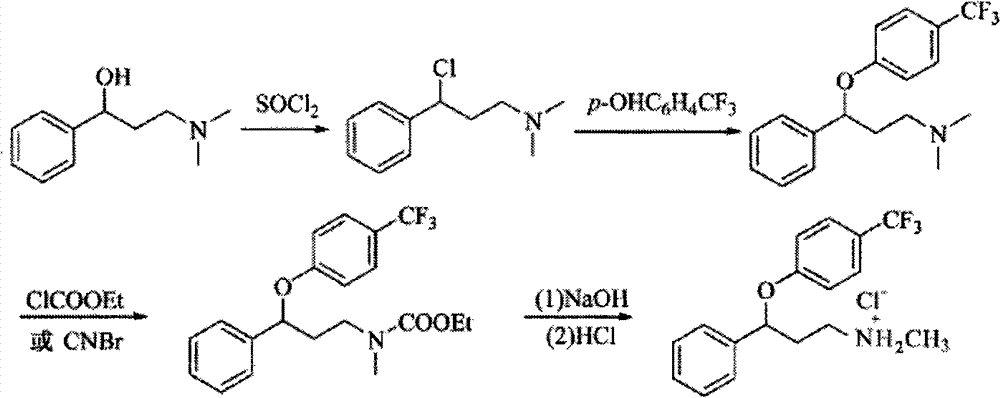
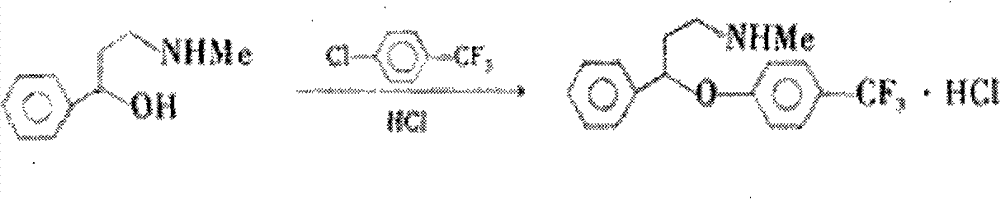
Method for preparing antidepressant fluoxetine
InactiveCN101962328AReduce pollutionAvoid introducingOrganic compound preparationAmino-hyroxy compound preparationP-chlorobenzotrifluorideAminolysis
The invention relates to a method for preparing antidepressant fluoxetine, and belongs to the method for preparing a medicinal compound. The technical scheme in the invention comprises the following three steps of aminolysis, catalytic hydrogenation and aromatic etherification reaction, wherein the three steps are performed in one pot without separation. In the method, the conventional chemical reduction method is replaced by the catalytic dehydration process, and the used reducing agent is hydrogen gas, so that the environmental pollution is reduced; the methylamine is gaseous methylamine, so that the reaction conversion rate is improved, and other substances, such as water and the like, are prevented from being introduced into a reaction system; and p-chlorobenzotrifluoride is added dropwise in the etherification reaction, so that the reaction speed can be conveniently controlled, the yield of a target product is improved, and the possibility of catalyst poisoning is reduced.
Owner:WENZHOU UNIVERSITY
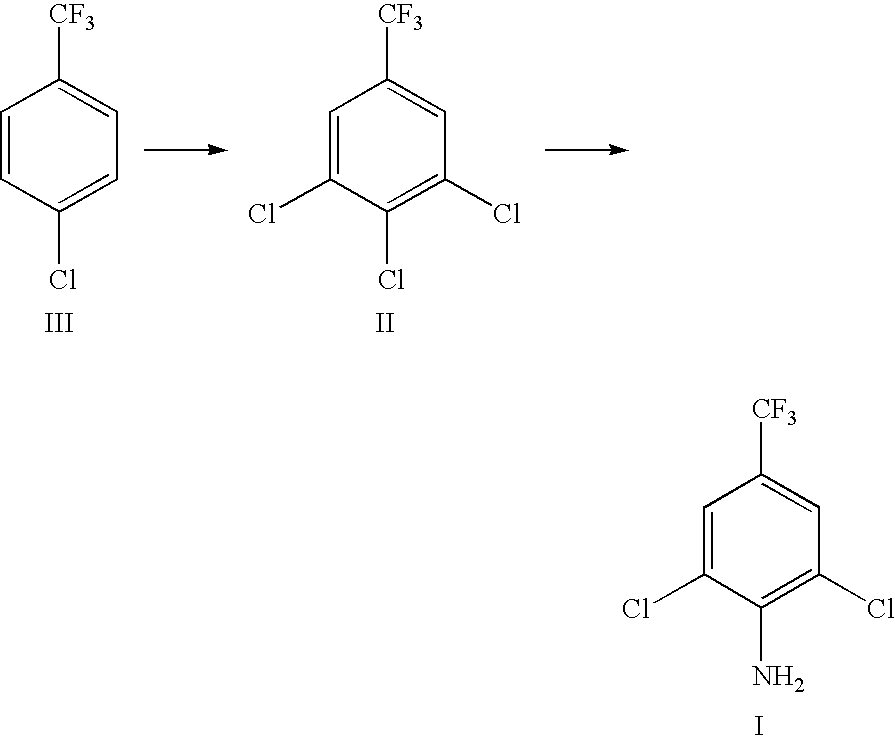
Preparation method of 2, 6-dichlor-4-trifluoromethyl aniline
ActiveUS7777079B2High yieldHigh feasibilityOrganic compound preparationAmino compound preparationP-chlorobenzotrifluorideAniline
This invention is involved with a preparation method of 2,6-dichloro-4-trifluoromethyl-aniline. With this process, p-Chlorobenzotrifluoride is used as the starting material and subjected to halogenation reaction and ammoniation reaction and through separation of reaction products the desired 2,6-dichloro-4-trifluoromethyl-aniline is obtained. In addition, ammonia is recovered from the surplus ammonia water in ammoniation reaction. This applied invention in characterized by simple process, cheap and easy-available raw materials, high reaction yield and friendly environment.
Owner:ZHEJIANG WEIHUA CHEMICAL CO LTD
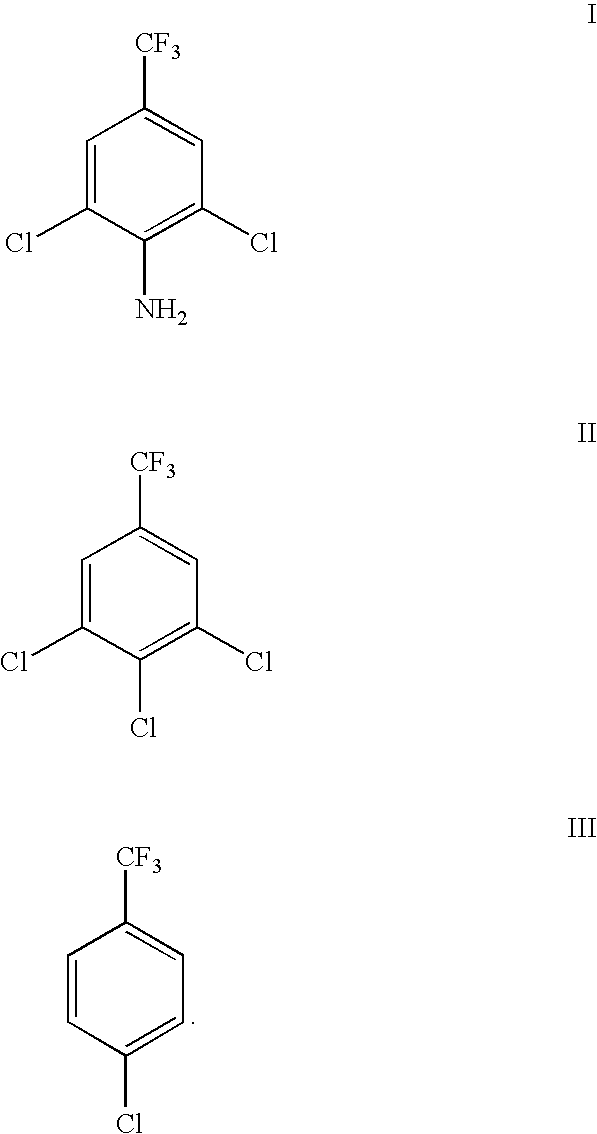
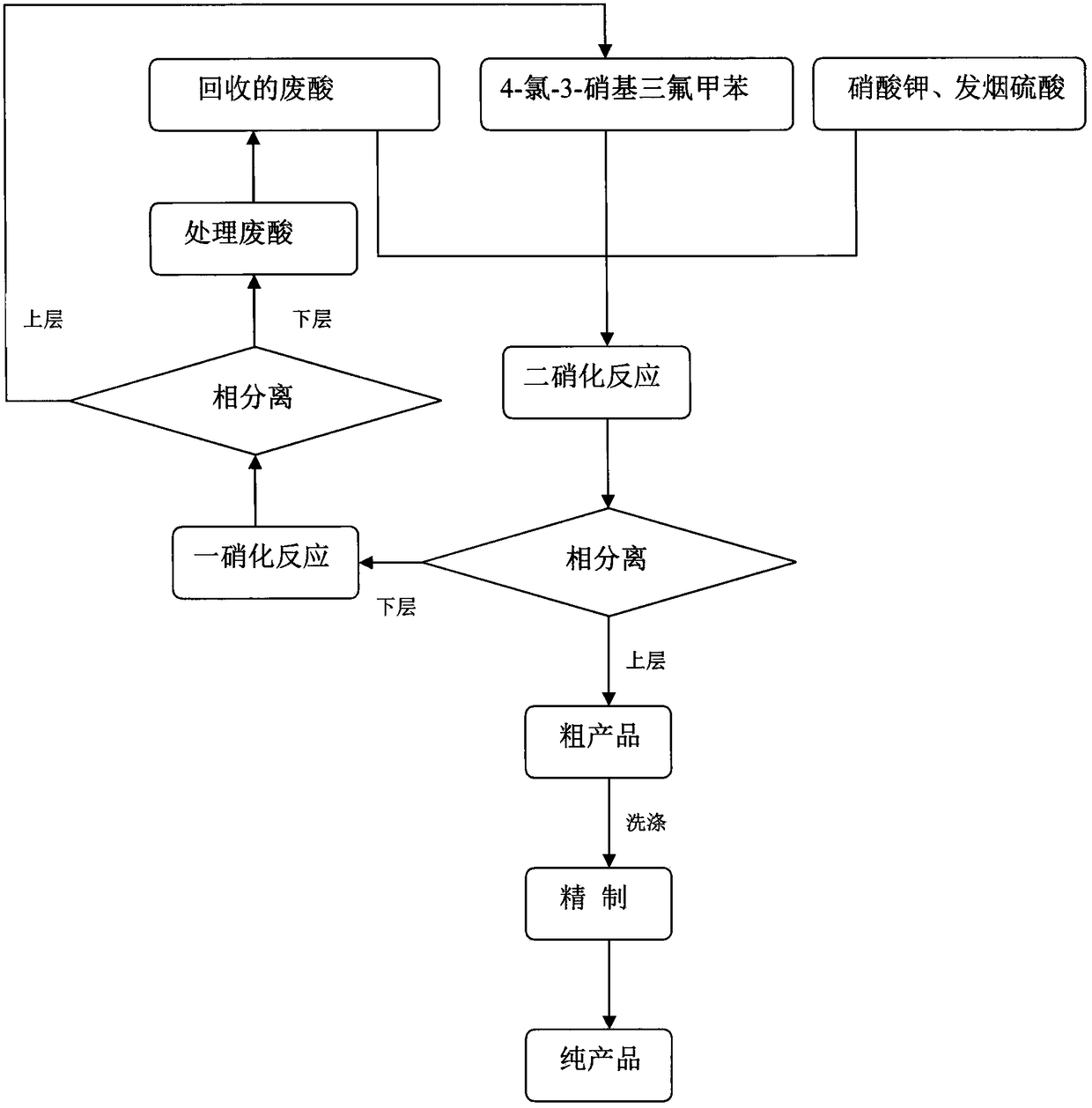
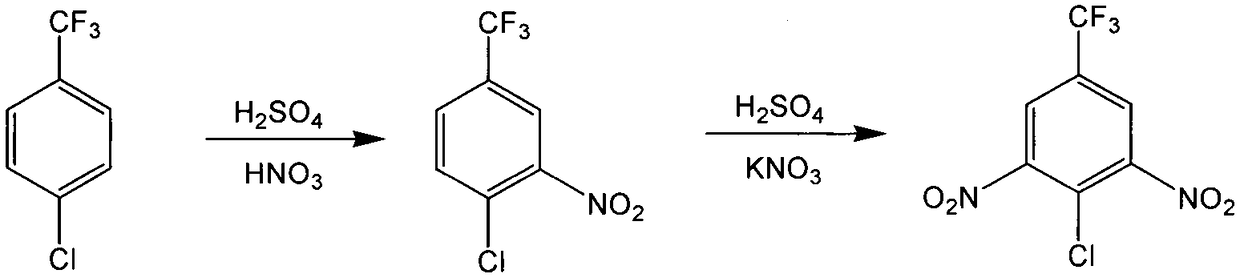
Novel nitration process of 3,5-binitro-4-chlorobenzotrifluoride
InactiveCN108383730AWide variety of sourcesEasy to useOrganic compound preparationNitro compound preparationP-chlorobenzotrifluorideNitration
The invention discloses a novel nitration process of 3,5-binitro-4-chlorobenzotrifluoride. The novel nitration process comprises the following steps: mixing nitrate, fuming sulfuric acid and a waste acid according to a ratio so as to obtain a mixed acid, performing a dinitration reaction with 4-chlorine-3-nitro trifluorotoluene in a reaction kettle, after the completion of a reaction, performing phase separation so as to obtain a semi-waste acid and a target compound, applying the semi-waste acid to mononitratio reaction on p-chlorobenzotrifluoride, performing phase separation so as to obtaina waste acid and a mononitratio compound, treating the waste acid in a waste acid kettle, performing a dinitration reaction on the treated waste acid, and further recycling the waste acid. Compared with the prior art, the novel nitration process is capable of reducing and eliminating pollution from sources, is an environmental-friendly chemical process and has good industrial application prospects.
Owner:JIANGSU DAHUA CHEM IND
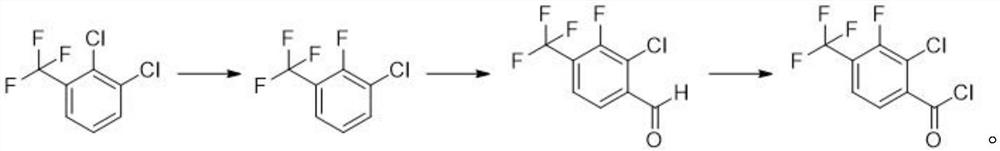
Synthesis method of 2-chloro-3-fluoro-4-trifluoromethyl benzoyl chloride
PendingCN113024372AAvoid harmEasy to recycleOrganic compound preparationCarbonyl compound preparationP-chlorobenzotrifluoridePtru catalyst
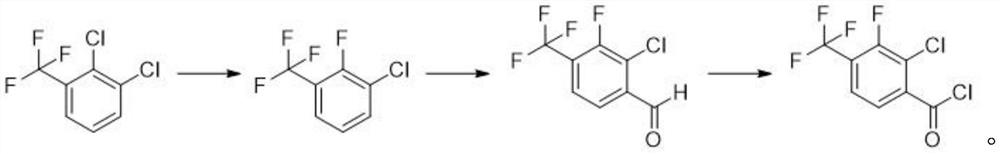
The invention discloses a synthesis method of 2-chloro-3-fluoro-4-trifluoromethyl benzoyl chloride, wherein the synthesis method comprises the steps: dissolving 2,3-dichlorobenzotrifluoride and potassium fluoride in a first solvent, and adding a first catalyst to obtain 2-fluoro-3-chlorobenzotrifluoride; dissolving an organic lithium reagent in a second solvent, reducing the temperature to -70 DEG C to -80 DEG C, adding 2-fluoro-3-chlorobenzotrifluoride, adding an acylation reagent, and thus obtaining 2-chloro-3-fluoro-4-trifluoromethyl benzaldehyde; and dissolving the 2-chloro-3-fluoro-4-trifluoromethyl benzaldehyde in a third solvent, and adding a chlorination reagent to obtain the 2-chloro-3-fluoro-4-trifluoromethyl benzoyl chloride. According to the invention, the 2-chloro-3-fluoro-4-trifluoromethyl benzoyl chloride is synthesized through a simple reaction, and flammable, explosive, highly toxic or difficult-to-store reagents commonly used in an existing synthesis method are not used in each reaction step, so that the harm to the environment and operators is avoided; and meanwhile, the yield of the product reaches 85% or above, and the purity is 95% or above.
Owner:内蒙古蓝科生物科技有限公司
Preparation method for 4-trifluoromethylbenzyl chloride
InactiveCN107793292AEasy to recycleThe route of raw materials is simpleOrganic compound preparationHydroxy compound preparationP-chlorobenzotrifluorideDistillation
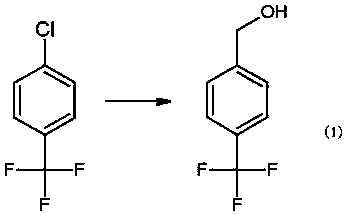
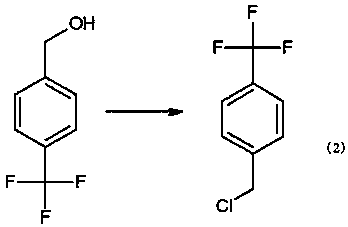
The invention relates to a preparation method for 4-trifluoromethylbenzyl chloride, wherein the preparation method comprises the specific steps: A, adding a proper amount of tetrahydrofuran, magnesium, iodine, bromoethane and p-chlorobenzotrifluoride into a reaction kettle, introducing nitrogen, stirring to rise the temperature to reflux, refluxing for 20 min-40 min, then controlling the temperature at 40 DEG C-60 DEG C, dropping p-trifluoromethyl chlorobenzene, after the dropping is finished, carrying out a heat preservation reaction for 1.5 h-3 h, then cooling to 5 DEG C-15 DEG C, adding a proper amount of paraformaldehyde, then controlling the temperature at 0 DEG C-60 DEG C, carrying out a reaction for 5 h-7 h, then recycling tetrahydrofuran, and carrying out reduced pressure distillation to obtain 4-trifluoromethylbenzyl alcohol; and B, adding a proper amount of hydrochloric acid and 4-trifluoromethylbenzyl alcohol into another reaction kettle, stirring to rise the temperature toreflux, carrying out a heat preservation reaction for 20 h-30 h, cooling to room temperature, stratifying, dehydrating, and carrying out reduced pressure distillation to obtain 4-trifluoromethylbenzylchloride. The preparation method for 4-trifluoromethylbenzyl chloride is simple in route and high in yield.
Owner:江苏万隆科技有限公司
Method for preparing antidepressant fluoxetine
InactiveCN101962328BReduce pollutionAvoid introducingOrganic compound preparationAmino-hyroxy compound preparationP-chlorobenzotrifluorideAminolysis
The invention relates to a method for preparing antidepressant fluoxetine, and belongs to the method for preparing a medicinal compound. The technical scheme in the invention comprises the following three steps of aminolysis, catalytic hydrogenation and aromatic etherification reaction, wherein the three steps are performed in one pot without separation. In the method, the conventional chemical reduction method is replaced by the catalytic dehydration process, and the used reducing agent is hydrogen gas, so that the environmental pollution is reduced; the methylamine is gaseous methylamine, so that the reaction conversion rate is improved, and other substances, such as water and the like, are prevented from being introduced into a reaction system; and p-chlorobenzotrifluoride is added dropwise in the etherification reaction, so that the reaction speed can be conveniently controlled, the yield of a target product is improved, and the possibility of catalyst poisoning is reduced.
Owner:WENZHOU UNIV
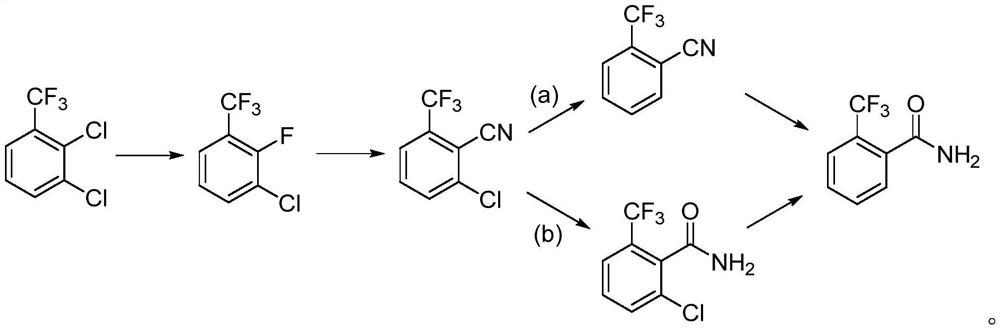
Synthesis method of 2-trifluoromethyl benzamide
PendingCN113698315ARaw materials are cheap and easy to getEasy to removeOrganic compound preparationPreparation by halogen replacementP-chlorobenzotrifluorideCyanogen fluoride
The invention discloses a synthesis method of 2-(trifluoromethyl) benzamide. The method comprises the following steps: by taking 2,3-dichlorobenzotrifluoride as a raw material, carrying out fluorination and cyano substitution to prepare 2-fluoro-6-cyano benzotrifluoride; and carrying out hydrogenation dechlorination and then hydrolysis on the 2-fluoro-6-cyano trifluorotoluene, or carrying out hydrolysis and then hydrogenation dechlorination on the 2-fluoro-6-cyano trifluorotoluene to prepare the 2-trifluoromethyl benzamide. The 2-trifluoromethyl benzamide is synthesized through simple reaction, and the method has the characteristics that the raw materials are cheap and easy to obtain, and an isomer of the intermediate 2-fluoro-3-chloro-trifluorotoluene does not participate in subsequent reaction and is easy to remove. In the steps, common flammable, explosive, highly toxic or difficult-to-preserve reagents in the existing synthesis method are not used, so that the harm to the environment and operators is avoided; meanwhile, the total yield of the product reaches 67% or above, and the purity is 97% or above.
Owner:内蒙古蓝科生物科技有限公司
Nifedipine drug intermediate o-nitrobenzaldehyde synthesis method
InactiveCN108238950AReduction of intermediate links in the reactionShort reaction timeOrganic chemistryOrganic compound preparationNitrobenzeneSodium nitrate
The invention discloses a nifedipine drug intermediate o-nitrobenzaldehyde synthesis method, which comprises: adding 3-bromo-2-(1-hydroxyethyl)-nitrobenzene and a sodium nitrate solution to a reactioncontainer, controlling the stirring speed, reducing the temperature of the solution, carrying out a reaction for 60-80 min, adding a dichlorobutene solution, adding rhodium trichloride in batches, continuously carrying out the reaction, layering the solution, extracting multiple times with a chlorodibromomethane solution, extracting multiple times with a thionyl chloride solution, washing multiple times with a potassium nitrate solution, separating to obtain the oil layer, dehydrating with a dehydrating agent, and re-crystallizing in a 3-chlorobenzotrifluoride solution to obtain the finishedproduct o-nitrobenzaldehyde.
Owner:CHENGDU QIESITE TECH CO LTD
Preparation method of 2-amino-5-chlorobenzotrifluoride
InactiveCN111116379AWill not cause pollutionIncrease productivityOrganic compound preparationAmino compound preparationP-chlorobenzotrifluoridePtru catalyst
The invention discloses a preparation method of 2-amino-5-chlorobenzotrifluoride, and belongs to the technical field of compound synthesis, and the preparation method comprises the following steps: 1,mixing m-chlorobenzotrifluoride, sulfuric acid and nitric acid together for nitration reaction; 2, separating the nitrified mixture in the step 1, collecting a reaction mixture, and removing waste acid at the same time; 3, adding sodium carbonate and water into the reaction mixture collected in the step 2 for neutralization; 4, adding ethanol into the neutralized mixture obtained in the step 3 for recrystallization; 5, drying the mixture recrystallized in the step 4; 6, adding hydrogen and a catalyst into the mixture dried in the fifth step for reduction; 7, carrying out reduced pressure distillation on the mixture reduced in the step 6 to obtain the finished product; 8, packaging the finished product obtained in the step 7. The method has the beneficial effects that the production efficiency is high, and environmental pollution cannot be caused after intermediate products are converted.
Owner:JIANGSU YONGCHUANG PHARMA TECH CO LTD
Synthetic method of p-trifluoromethylaniline
InactiveCN101298421BLow priceNo emissionsPhysical/chemical process catalystsOrganic compound preparationP-chlorobenzotrifluorideOrganic solvent
Owner:太仓中化环保化工有限公司
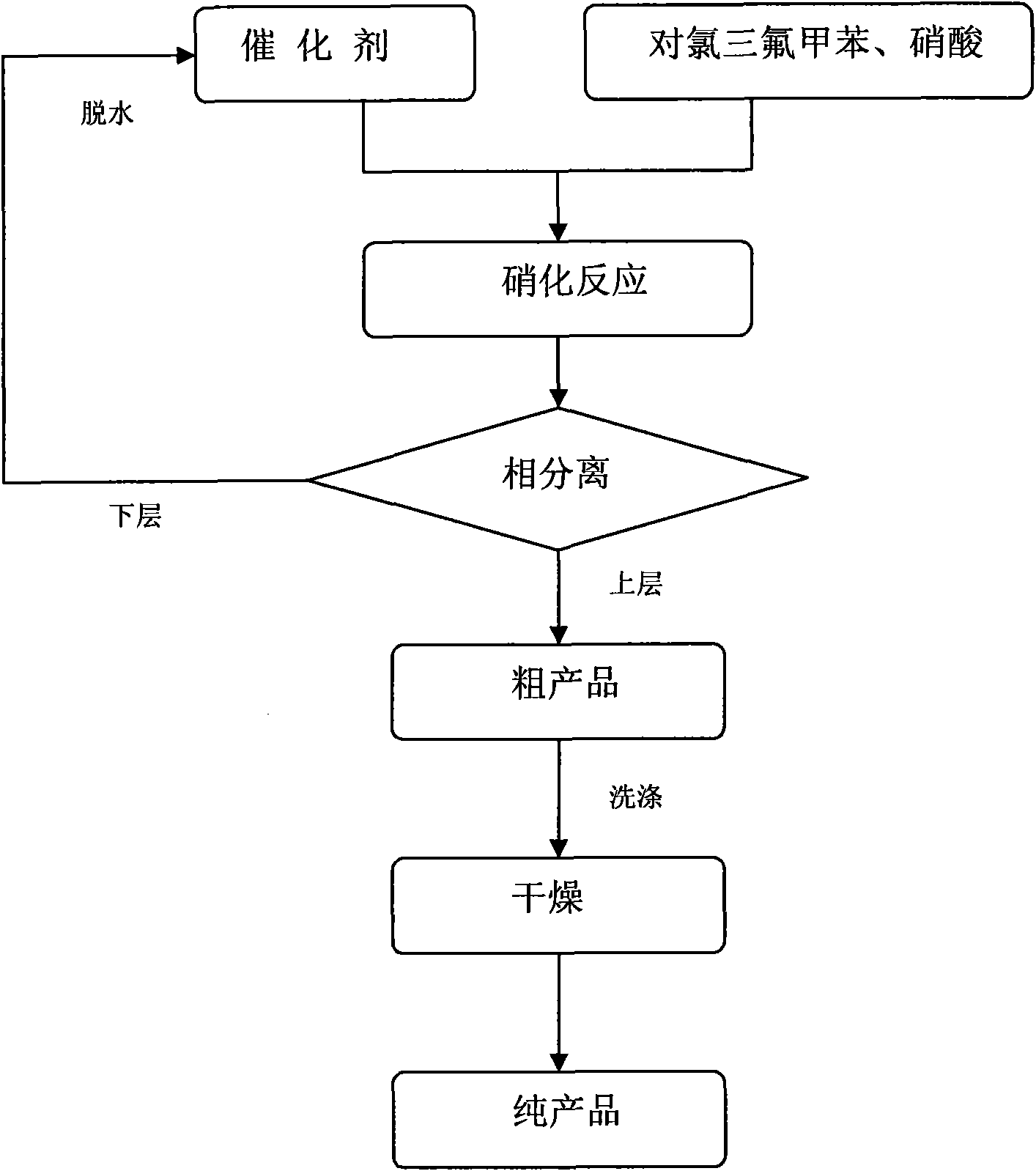
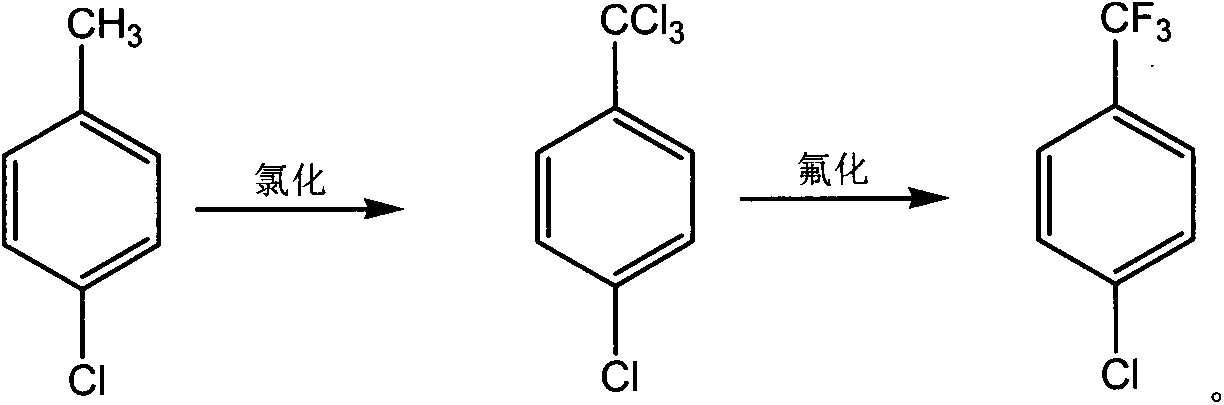
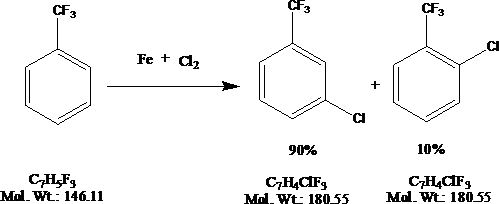
Novel synthesis method of chlorobenzotrifluoride
InactiveCN110655449AFewer chlorination side reactionsHigh purityPreparation by halogen replacementOrtho-chlorotolueneP-chlorobenzotrifluoride
The invention discloses a novel synthesis method of chlorobenzotrifluoride, wherein o-chlorotoluene is used as a raw material, copper chloride or cuprous chloride is used as a catalyst, and o-chlorobenzotrifluoride is synthesized through two steps of chlorination and fluorination. Compared with the method in the prior art, the method of the invention has the following characteristics that raw materials are subjected to heating dehydration treatment in the dehydration kettle, so that the chlorination side reactions are few, the product purity is high, the graded chlorination device does not need to be additionally arranged, the technological process is simple, and the operation is convenient. According to the chlorination process of the invention, the catalyst is added in batches to increase the efficiency of the catalyst, so that the by-product generated by thechlorination reaction and the emission of waste gas in the production are greatly reduced, and the emission of pollutants in the production process is reduced.
Owner:JIANGSU FENGHUA CHEM IND
Preparation method of 2, 4-dichlorobenzotrifluoride
PendingCN114105726AImprove the effective rateReduce generationPreparation by halogen replacementHydrogen fluorideP-chlorobenzotrifluoride
The invention discloses a preparation method of 2, 4-dichlorobenzotrifluoride, which comprises the following steps: (1) nuclear chlorination: taking parachlorotoluene as a raw material, carrying out multiple times of rectification and separation on the raw material nuclear chlorination in a rectification tube type integrated reactor under the action of a Lewis acid catalyst, and carrying out rectification and separation on the product to obtain a mixture of 2, 4-dichlorotoluene and 3, 4-dichlorotoluene; and (2) side chain chlorination: carrying out side chain chlorination on the mixture of the 2, 4-dichlorotoluene and the 3, 4-dichlorotoluene under the action of a photochlorination catalyst and an inhibitor to obtain a mixture of the 2, 4-dichlorotrichlorotoluene and the 3, 4-dichlorotrichlorotoluene. And (3) fluorination: reacting the side chain chlorination product with hydrogen fluoride under the action of a fluorination catalyst, after directional side chain fluorination, removing acid and moisture in the reaction liquid, and rectifying and separating to obtain the product 2, 4-dichlorobenzotrifluoride.
Owner:JIANGSU SANMEI CHEM
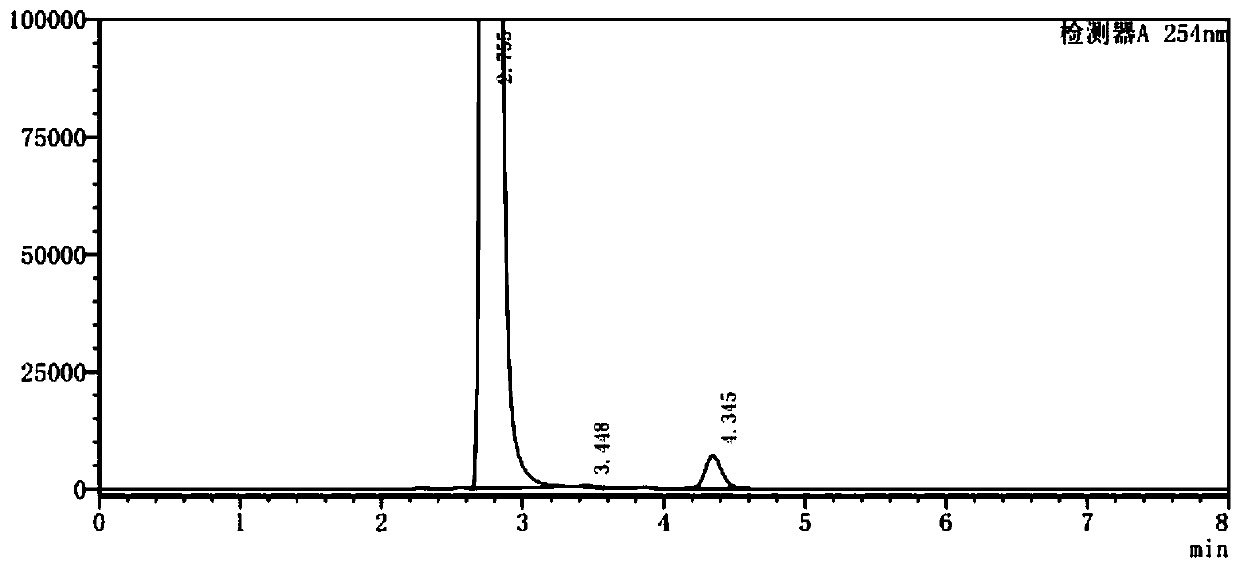
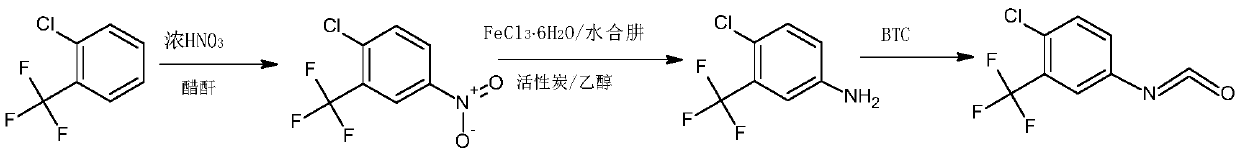
The synthetic method of 4-chloro-3-(trifluoromethyl)benzene isocyanate
ActiveCN110885298BReduce riskAvoid it happening againIsocyanic acid derivatives preparationOrganic compound preparationMethylanilineAcetic anhydride
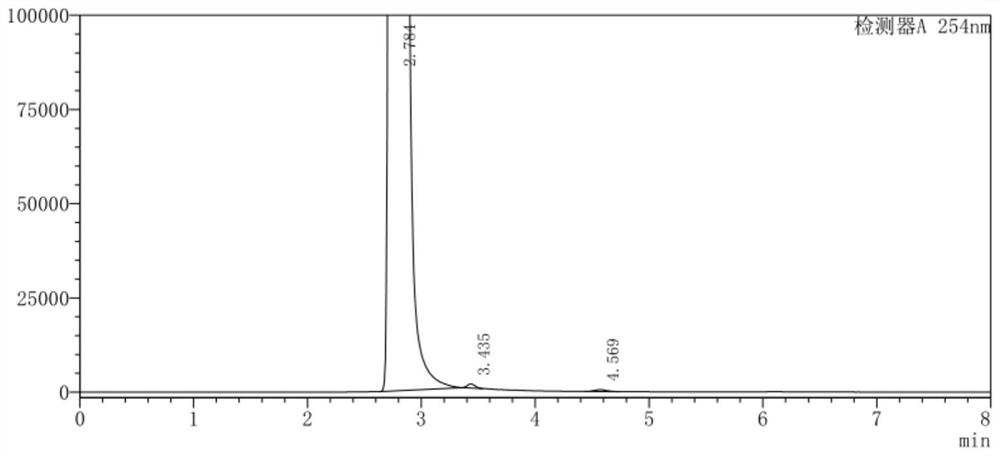
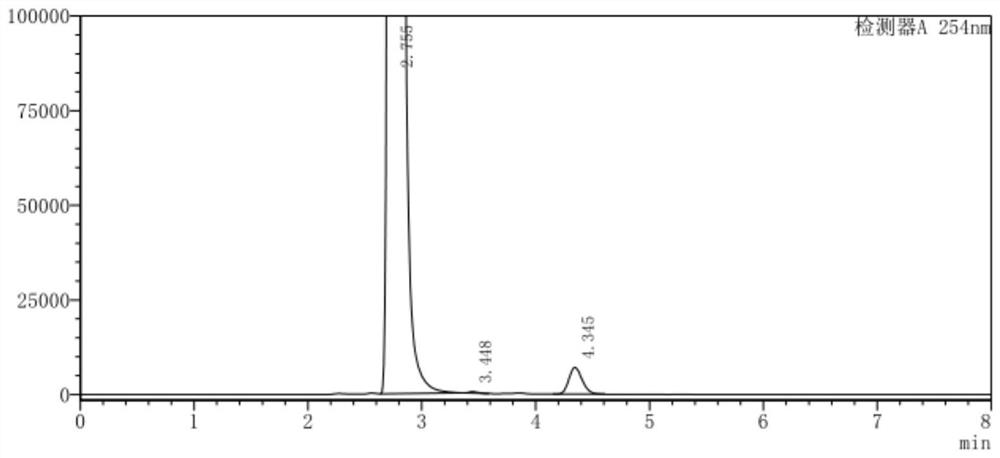
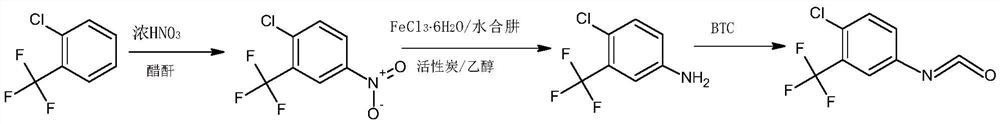
The invention belongs to the technical field of medicine, and in particular relates to a synthetic method of 4-chloro-3-(trifluoromethyl)phenylisocyanate. The reaction of o-chlorobenzotrifluoride, acetic anhydride and concentrated nitric acid gives 4-nitro-2-trifluoromethyl chlorobenzene, 4-nitro-2-trifluoromethyl chlorobenzene, activated carbon, FeCl 3 ·6H 2 O reacts with hydrazine hydrate to obtain 4-chloro-3-trifluoromethylaniline, and 4-chloro-3-trifluoromethylaniline, triphosgene and catalyst react to obtain 4-chloro-3-(trifluoromethyl) benzene isocyanate . The nitration process of the present invention uses the acetic anhydride / concentrated nitric acid system to replace the traditional nitric acid / sulfuric acid mixed acid system, and the strong nitration effect of acetyl nitrate can complete the reaction at a lower temperature, with low risk and less nitration impurities; the reduction process uses FeCl 3 ·6H 2 The O / activated carbon / hydrazine hydrate system replaces the traditional iron powder reduction process, which avoids the generation of a large amount of iron sludge and waste, and reduces the pressure on environmental protection.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Novel fluorination process of p-chlorobenzotrifluoride
InactiveCN110483238ANo pollution in the processLow costPreparation by halogen replacementP-chlorobenzotrifluorideSolvent free
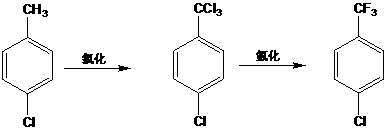
The invention discloses a novel fluorination process of p-chlorobenzotrifluoride. P-chlorotoluene is used as a raw material, and 4-chlorobenzotrifluoride is synthesized through two steps of chlorination and fluorination. Compared with the prior art, the fluorination process has the advantages of no catalyst, lower cost than other organic catalysts or noble metal catalysts, convenience in recoveryand no pollution to the environment; a solvent-free fluorination process is adopted, so that the emission of by-products and waste gas of a solvation reaction is greatly reduced, and the emission of pollutants in the production process is reduced.
Owner:JIANGSU DAHUA CHEM IND
Clean nitration reaction of p-chlorobenzotrifluoride under catalysis of degradable functional ionic liquid
ActiveCN102249927BWide variety of sourcesEasy to manufactureOrganic-compounds/hydrides/coordination-complexes catalystsNitro compound preparationP-chlorobenzotrifluorideQuaternary ammonium cation
The invention discloses a new method of clean nitration reaction of p-chlorobenzotrifluoride under catalysis of degradable functional ionic liquid. Biodegradable ionic liquid of quaternary ammonium cation structure is used as a catalyst, p-chlorobenzotrifluoride and nitric acid are used as raw materials, and 4-chloro-3-nitrobenzotrifluoride is obtained by nitration reaction under the action of the catalyst. Compared with the prior art, the method has the advantages that: (1) because the degradable ionic liquid of quaternary ammonium cation structure is adopted, the raw material source is extensive, and the preparation is convenient; the catalyst has high activity, low consumption and water stability, is not inactivated and can be recycled; (2) the ionic liquid is biodegradable and environment-friendly; and (3) because the ionic liquid is used for substituting concentrated sulfuric acid, the method is an environment-friendly chemical process, and has good industrialized application prospect.
Owner:内蒙古大中实业化工有限公司
Synthetic method of 4-chloro-3-(trifluoromethyl)phenyl isocyanate
ActiveCN110885298AReduce riskAvoid it happening againIsocyanic acid derivatives preparationOrganic compound preparationMethylanilineP-chlorobenzotrifluoride
The invention belongs to the technical field of medicines, and particularly relates to a synthetic method of 4-chloro-3-(trifluoromethyl)phenyl isocyanate. Chlorobenzotrifluoride, acetic anhydride andconcentrated nitric acid react to obtain 4-nitro-2-chlorotrifluoromethylbenzene, the 4-nitro-2-chlorotrifluoromethylbenzene, activated carbon, FeCl36H2O and hydrazine hydrate react to obtain 4-chloro-3-trifluoromethylaniline, and 4-chloro-3-trifluoromethylaniline, triphosgene and a catalyst react to obtain 4-chloro-3-(trifluoromethyl)phenyl isocyanate. According to the invention, in a nitration process, an acetic anhydride / concentrated nitric acid system is used for replacing a traditional nitric acid / sulfuric acid mixed acid system, the reaction can be completed at a low temperature by utilizing the strong nitration effect of acetyl nitrate, the risk is low, and more nitration impurities are few; in a reduction process, a FeCl36H2O / activated carbon / hydrazine hydrate system is used for replacing a traditional iron powder reduction process, so that generation of a large amount of iron mud waste residues is avoided, and the environmental protection pressure is reduced.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Injection needle coating material and application thereof in surface treatment of peripheral blood taking needle head
PendingCN112957542AImprove the lubrication effectRelieve painSurgeryDiagnostic recording/measuringCelluloseP-chlorobenzotrifluoride
The invention discloses an injection needle coating material and application thereof in surface treatment of a peripheral blood taking needle head, and relates to the technical field of biomedical materials. According to the injection needle coating material, an injection needle head is soaked in a coating solution to form a coating on the surface of the needle head, and the coating solution is prepared from the following raw materials in parts by weight: 1 to 4 parts of modified silicone oil, 20 to 30 parts of 4-chlorobenzotrifluoride, 15 to 30 parts of ethyl acetate, 2 to 4 parts of hydroxypropyl methyl cellulose, 3 to 8 parts of dimethylformamide, 0.5 to 2 parts of fucosan sulfate and 0.1 to 1 part of luteolin-5-O-glucoside. The coating material prepared by the invention has good cohesiveness, is not easy to fall off when being coated on the surface of the needle head, and can greatly reduce the pain of a sick body during puncture; and the coating material has an excellent anticoagulant effect, and can relieve the problem that the blood coagulation of a patient is fast and is not beneficial to follow-up test.
Owner:PROMISEMED HANGZHOU MEDITECH
Method for synthesizing 2, 5-dichlorobenzotrifluoride through continuous flow catalytic chlorination
PendingCN113831214AReduce generationHigh yieldHalogenated hydrocarbon preparationP-chlorobenzotrifluoridePtru catalyst
The invention provides a method for synthesizing 2, 5-dichlorobenzotrifluoride through continuous flow catalytic chlorination, and belongs to the field of synthesis processes. According to the method, o-chlorobenzotrifluoride and chlorine are taken as raw materials, and the 2, 5-dichlorobenzotrifluoride is synthesized in a continuous flow reactor with high selectivity in the presence of a catalyst. Compared with the traditional method, the synthesis method disclosed by the invention has the advantages that the selectivity is higher, the generation of byproducts is greatly reduced, the reaction time is shortened, the production efficiency is improved, the 2, 5-dichlorobenzotrifluoride with high yield and high purity is obtained, and the industrial application prospect is very good.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Novel synthetic method of halogenated aromatic hydrocarbon
InactiveCN110668911AFewer chlorination side reactionsHigh purityPreparation by halogen replacementP-chlorobenzotrifluoridePtru catalyst
The invention discloses a novel synthetic method of halogenated aromatic hydrocarbon, wherein p-chlorotoluene is used as a raw material, copper chloride or cuprous chloride is used as a catalyst, andp-chlorobenzotrifluoride is synthesized through two steps of chlorination and fluorination. Compared with the method in the prior art, the method of the invention has the following characteristics that raw materials are subjected to heating dehydration treatment in a dehydration kettle, so that the chlorination side reactions are few, the product purity is high, the grading chlorination device does not need to be additionally arranged, the technological process is simple, and the operation is convenient; and by adding the catalyst in batches in the chlorination process, the use efficiency of the catalyst is improved, so that the emission of the byproducts and the waste gas generated by the chlorination reaction in production is greatly reduced, and the emission of pollutants in the production process is reduced.
Owner:JIANGSU FENGHUA CHEM IND
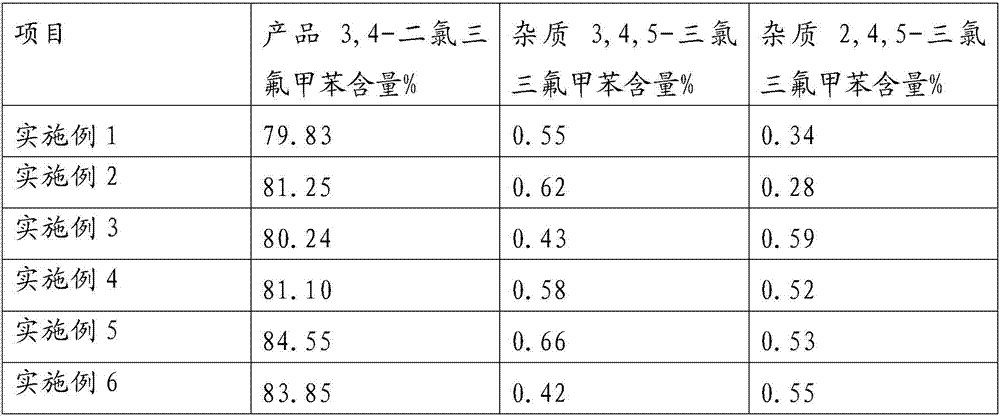
New process for synthesizing 3,4,5-trichlorobenzotrifluoride
InactiveCN110655441AAvoid heavy useHarm reductionHalogenated hydrocarbon preparationP-chlorobenzotrifluorideBiochemical engineering
The invention discloses a new process for synthesizing 3,4,5-trichlorobenzotrifluoride, wherein p-chlorobenzotrifluoride is used as a raw material, andchlorination, rectification and neutralization are performed to obtain the 3,4,5-trichlorobenzotrifluoride. Compared with the method in the prior art, the method of the invention has the following characteristics that the used raw materials avoid alarge amount of sulfuric acid and nitric acid, so that the corrosion to equipment is small, the pollution is greatly reduced, and the harm to the environment is reduced; the used raw material p-chlorobenzotrifluoride is wide in source and low in price, so that the production cost of the target compound can be reduced, and the economic benefit is improved; and by adjusting the reaction conditions,the content of the 3,4,5-trichlorobenzotrifluoride achieves 8-10%, and the 3,4-dichlorobenzotrifluoride is separated from the 3,4,5-trichlorobenzotrifluoride by rectification, such that the operationis simple and convenient.
Owner:JIANGSU FENGHUA CHEM IND
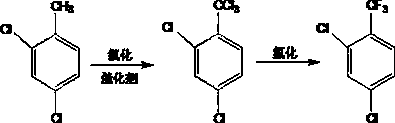
P-chlorobenzotrifluoride synthesizing method
InactiveCN103787825AFewer chlorination side reactionsHigh purityPreparation by halogen replacementState of artP-chlorobenzotrifluoride
The invention discloses a p-chlorobenzotrifluoride synthesizing method. P-chlorotoluene is used as a raw material, and p-chlorobenzotrifluoride is synthesized through chlorination and fluorination. Compared with the prior art, in the method, a dehydration kettle is used for performing thermal dehydration processing on the raw material, side reactions of chlorination are few, the product is high in purity, no grading chlorination device needs to be added, and the technological process is simple and convenient to operate; according to the method, in the chlorination process, the mode that catalysts are added in batches is adopted, so that the use efficiency of the catalysts is improved, therefore, emission of by-products and exhaust gas generated by chlorination in production is reduced significantly, and therefore emission of pollutants in the production process is reduced.
Owner:JIANGSU DAHUA CHEM IND
New synthesis process of m-chlorobenzotrifluoride
ActiveCN111848332ALow priceShort stepsPreparation by dehalogenationHalogenated hydrocarbon separation/purificationP-chlorobenzotrifluoridePtru catalyst
The invention discloses a new synthesis process of m-chlorobenzotrifluoride, and relates to the technical field of synthesis of m-chlorobenzotrifluoride, in particular to the new synthesis process ofm-chlorobenzotrifluoride. The process comprises the following steps of: S1, weighing each component; S2, conducting replacement treatment; S3, performing temperature control; S4, conducting sampling and analyzing experimental data; S5, performing standing for layering; S6, conducting rectification; and S7, repeating the experiment. According to the invention, 3, 4-dichlorobenzotrifluoride is usedas a raw material, and due to the fact that trifluoromethyl has different influences on chlorine at different positions on a benzene ring, in the presence of an efficient catalyst, during hydrogenation, high selectivity can be achieved; cyclohexane, ethanol and trifluorotoluene are used as the solvent, triethylamine and potassium hydroxide are taken as the acid-binding agent, raney nickel, ruthenium carbon and palladium carbon are taken as the catalyst, hydrogen is introduced under a pressure of 0.5-2.0MPa and at a temperature of 60-100DEG C, and after hydrodechlorination, the solvent is removed, and the m-chlorobenzotrifluoride content reaches 99% or above through rectification separation and purification.
Owner:上海嘉化科技有限公司
2,4-dichlorobenzotrifluoride preparation method
InactiveCN110655447AWide variety of sourcesLow pricePreparation by halogen replacementChemical recyclingP-chlorobenzotrifluoridePtru catalyst
The invention discloses a 2,4-dichlorobenzotrifluoridepreparation method, wherein the target compound is obtained by using 2,4-dichlorotoluene as a raw material and using a mixture of cuprous chlorideand copper powder as a catalyst through a two-step reaction of chlorination and fluorination. Compared with the method in the prior art, the method of the invention has the following advantages thatthe chlorination side reactions are less, the isomer of 2,4-dichlorobenzotrifluoride cannot be generated in the reaction process, the product purity is high, the raw material sources are wide, the catalyst is low in price and convenient to recover, the production cost of the target compound is reduced, the economic benefit is increased, and environment pollution cannot be generated.
Owner:JIANGSU FENGHUA CHEM IND
Preparation method of 2-fluoro-4-cyano trifluorotoluene
PendingCN114230486AIncrease supplyLow priceOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by cyanide reactionP-chlorobenzotrifluoridePtru catalyst
The invention relates to the field of synthesis of medical intermediates, in particular to a preparation method of 2-fluoro-4-cyanobenzotrifluoride. The preparation method comprises the following steps: activating zinc powder, preparing a catalyst tetrakis (triphenylphosphine) nickel, adding a main reactant 2-fluoro-4-chlorobenzotrifluoride, and reacting to obtain the 2-fluoro-4-cyanobenzotrifluoride. The raw materials adopted in the method are large in supply in the market and low in price. In the reaction process, full reaction conversion with the prepared activated zinc powder can be realized, so that full conversion is ensured, and generation of impurities is reduced. The conversion yield and the product purity are improved. In the whole process, nitrogen is continuously introduced to ensure that combustibles in the reaction are cooled to a safe state under the protection of inert gas, and meanwhile, air suck-back can be prevented.
Owner:阜新金特莱氟化学有限责任公司
Novel nitrating process of 3-nitro-4-chlorobenzotrifluoride
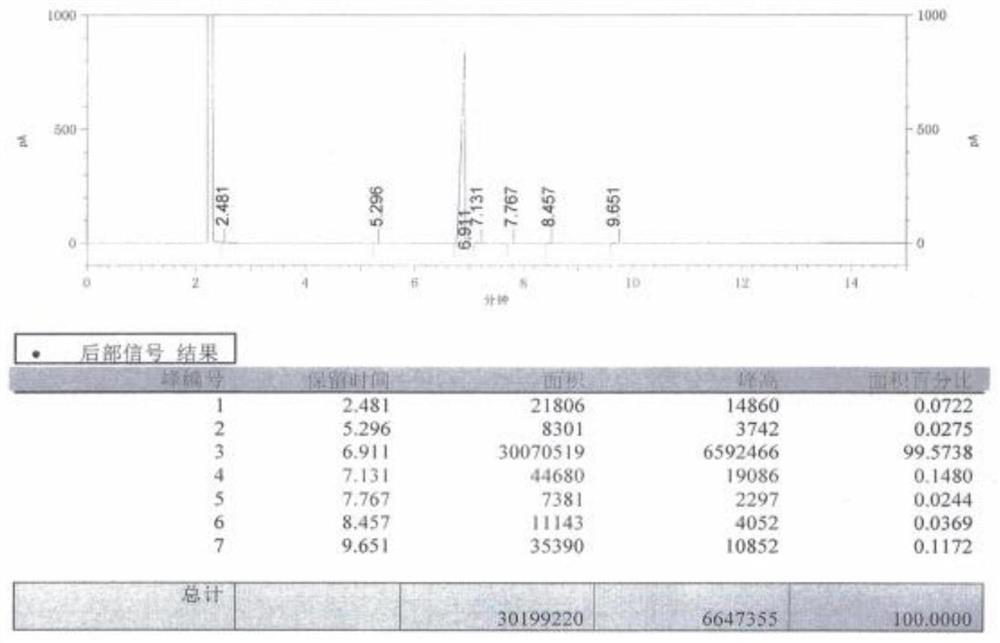
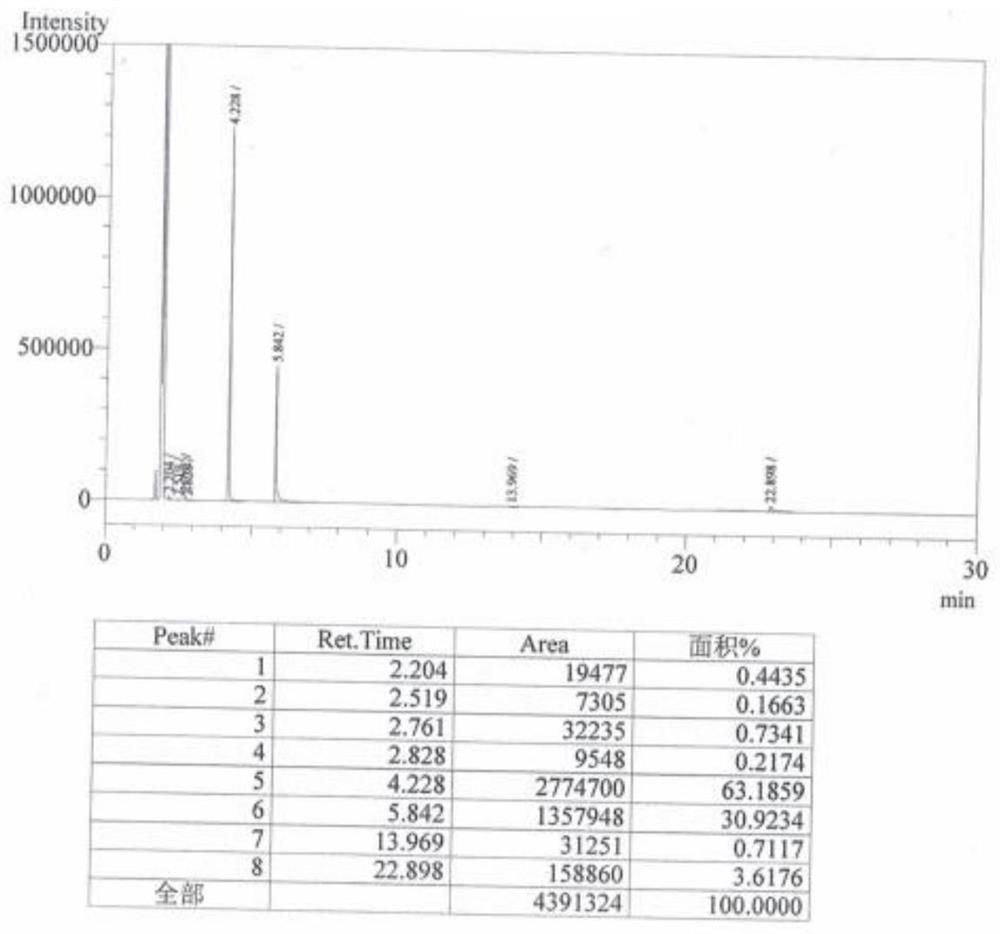
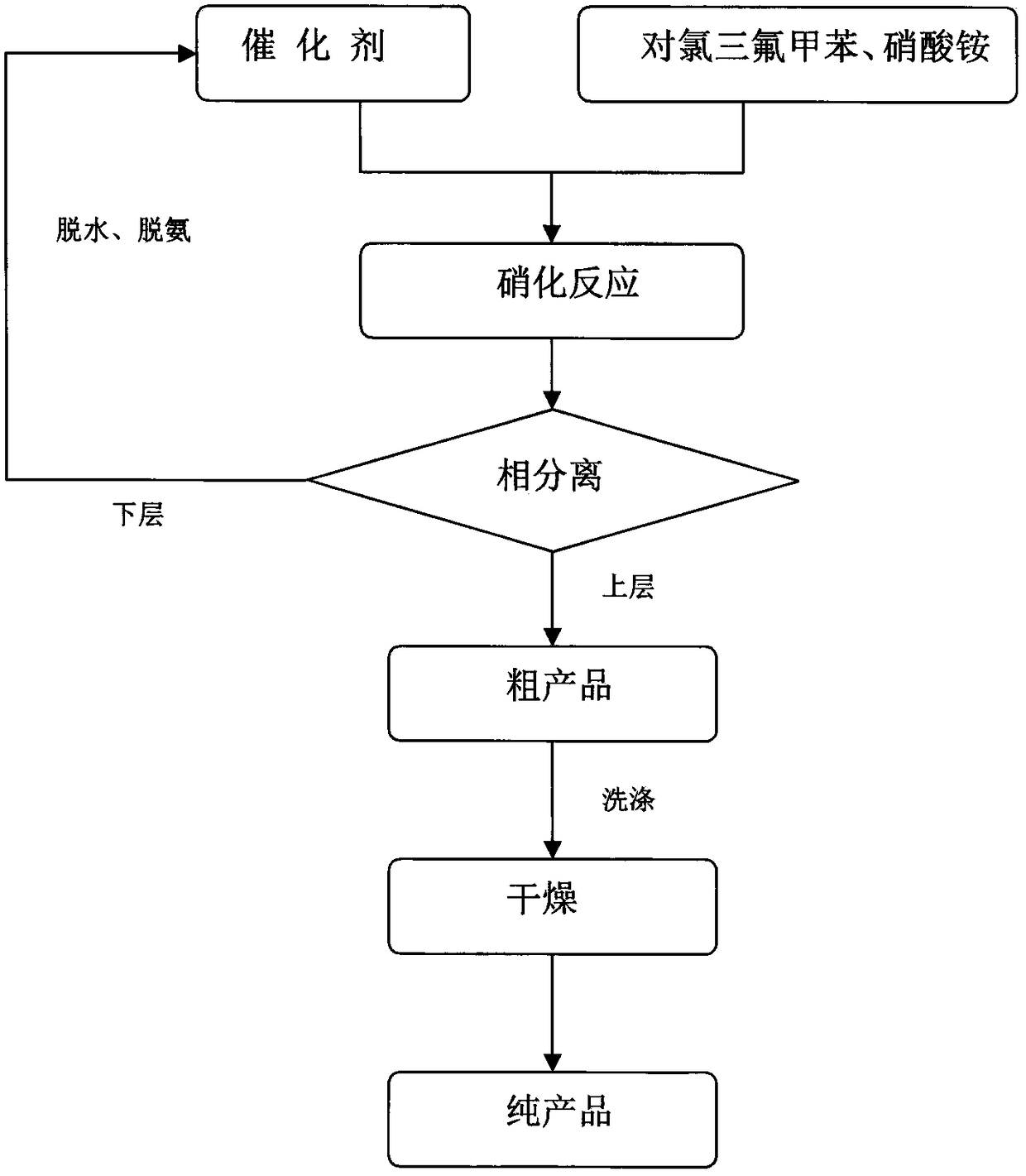
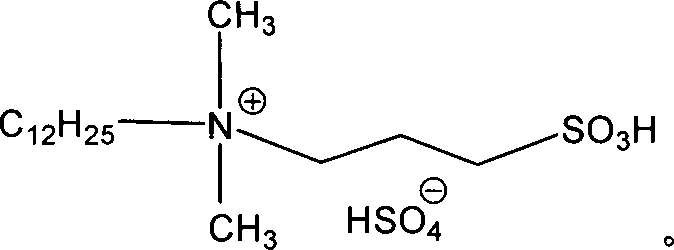
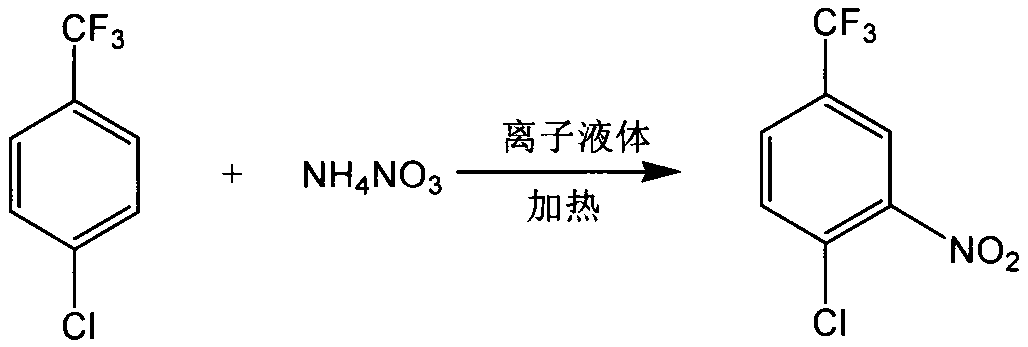
InactiveCN108484409AWide variety of sourcesEasy to useBulk chemical productionNitro compound preparationP-chlorobenzotrifluorideQuaternary ammonium cation
The invention discloses a novel nitrating process of 3-nitro-4-chlorobenzotrifluoride. A used catalyst is a biodegradable ionic liquid with a quaternary ammonium cation structure; raw materials are p-chlorobenzotrifluoride and ammonium nitrate; nitration reaction is carried out under the action of the catalyst, so that the 3-nitro-4-chlorobenzotrifluoride is obtained. Compared with the prior art,the novel nitrating process of the 3-nitro-4-chlortrifluorotoluene, provided by the invention, has the advantages that (1) ammonium nitrate is adopted to replace nitric acid, so that the 3-nitro-4-chlorobenzotrifluoride is wide in raw material source, convenient to use, and less in equipment corrosion; (2) the ion liquid can be biodegraded, so that the 3-nitro-4-chlorobenzotrifluoride is environmentally friendly; (3) the ion liquid replaces concentrated sulfuric acid, so that the novel nitrating process of the 3-nitro-4-chlortrifluorotoluene is an environment-friendly chemical process, and hasa favorable industrial application prospect.
Owner:JIANGSU DAHUA CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com