Novel synthesis method of chlorobenzotrifluoride
A technology of chlorinated benzotrifluoride and a synthetic method, which is applied in the field of preparation of pesticide chemical intermediate products, can solve the problems of low absorption utilization rate of chlorine gas, low reaction yield and other problems, and achieve convenient post-processing recovery, convenient operation, and reduction of The effect of pollutant emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
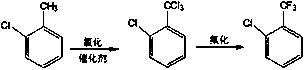
[0024] (1) Chlorination step: put 630g of o-chlorotoluene into a dehydration kettle, heat to 80°C to remove water, and obtain 629g of o-chlorotoluene, put it into a 3L chlorination reaction kettle, heat up to 110°C, add 31.5g of chlorinated ethylene Copper catalyst (added in 4 batches, each batch 8-10g), start to pass dry chlorine gas, flow 40-80 m 3 / h, then add the catalyst every 30 minutes, control the temperature at 110-112°C, and the reaction time is 20 hours. After sampling and analyzing the chlorinated material as qualified, stop the chlorine flow. The o-chlorotrichlorotoluene produced by chlorination is pumped into the chlorination tank for use, and the tail gas generated by the reaction is sent to the tail gas absorption device.
[0025] (2) Fluorination step: Press 4 moles of o-chlorotrichlorotoluene and 14 moles of anhydrous hydrogen fluoride into a 3L fluorination reaction kettle, open the frozen brine system, press hydrogen fluoride into the fluorination kettle w...
Embodiment 2
[0030] (1) Chlorination step: put 470kg of o-chlorotoluene into a dehydration kettle, heat to 80°C to remove water to obtain dry o-chlorotoluene, put it into a 3000L chlorination reaction kettle, raise the temperature to 110°C, add 25kg of chlorinated Copper catalyst (added in 4 batches, each batch 6-7kg), start to pass dry chlorine gas, the flow rate is 40-80 m 3 / h, then add the catalyst every 30 minutes, control the temperature at 110-112°C, and the reaction time is 20 hours. After sampling and analyzing the chlorinated material as qualified, stop the chlorine flow. The o-chlorotrichlorotoluene produced by chlorination is pumped into the chlorination tank for use, and the tail gas generated by the reaction is sent to the tail gas absorption device.
[0031] (2) Fluorination step: pump 690kg of o-chlorotrichlorotoluene into the high level tank for metering and put it into the fluorination kettle, open the frozen brine system, and press 250kg of anhydrous hydrogen fluoride i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com