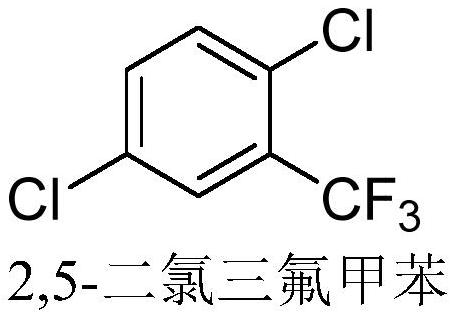
Method for synthesizing 2, 5-dichlorobenzotrifluoride through continuous flow catalytic chlorination
A technology of dichlorotrifluorotoluene, stream synthesis, applied in chemical instruments and methods, organic chemistry, halogenated hydrocarbon preparation and other directions, to achieve the effects of stable product quality, stable production process and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, utilize continuous flow reactor to synthesize the method for 2,5-dichlorobenzotrifluoride
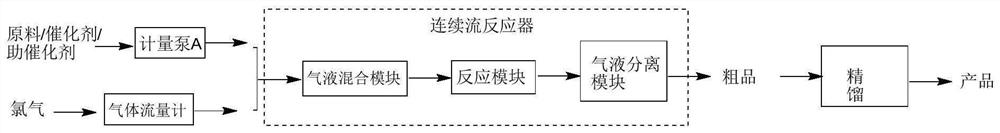
[0037] The continuous flow reactor includes a gas-liquid mixing module, a reaction module, and a gas-liquid separation module connected in sequence. The reaction scheme is as follows:
[0038]
[0039] The reaction operates as follows:
[0040] Take 50g of o-chlorobenzotrifluoride, mix with 5g of ferric chloride and 0.5g of bromobenzene evenly, transport it into the gas-liquid mixing module through a metering pump, and the flow rate is 10mL / min; transport chlorine gas into the gas-liquid mixing module through a gas flow meter, The flow rate is 6L / min. After staying in the gas-liquid mixing module for 0.5 minutes, enter the reaction module for a full reaction. The residence time in the reaction module is 10 minutes, and the temperature of the gas-liquid mixing module and the reaction module is set to 0°C. Then enter the gas-liquid separation module to collect t...
Embodiment 2
[0042] Embodiment 2, utilize continuous flow reactor to synthesize the method for 2,5-dichlorobenzotrifluoride
[0043] Referring to the method of Example 1, 2,5-dichlorobenzotrifluoride was synthesized by using a continuous flow reactor, the only difference being that no cocatalyst bromobenzene was added. The GC purity of the pure 2,5-dichlorotrifluorotoluene obtained in Example 2 was 98.5%, and the yield was 55%.
Embodiment 3
[0044] Embodiment 3, utilize continuous flow reactor to synthesize the method for 2,5-dichlorobenzotrifluoride
[0045] Referring to the method of Example 1, 2,5-dichlorobenzotrifluoride was synthesized by using a continuous flow reactor, the only difference being that neither the catalyst ferric chloride nor the promoter ferric chloride was added. The GC purity of the pure 2,5-dichlorobenzotrifluoride obtained in Example 3 was 95.5%, and the yield was 34%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com