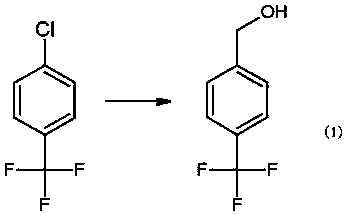
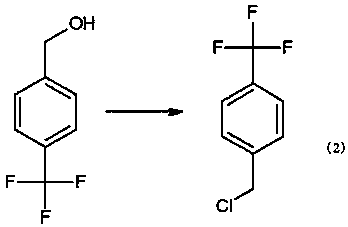
Preparation method for 4-trifluoromethylbenzyl chloride
A technology of p-trifluoromethylbenzyl chloride and p-chlorobenzotrifluoride, which is applied in the field of compound preparation, can solve the problems of low efficiency and high cost, and achieve the effects of reducing cost, short reaction process, and improving yield and production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The concrete steps of the preparation method of trifluoromethylbenzyl chloride of the present embodiment comprise:
[0017] A. Add 150kg of pretreated tetrahydrofuran, 7.3kg of dried magnesium particles, 30g of iodine, 1kg of bromoethane and 0.5kg of p-chlorobenzotrifluoride into the reaction kettle, slowly feed nitrogen, stir and heat up to reflux, and keep reflux After 30 minutes, the Grignard reagent was successfully initiated, then the temperature was controlled at 50°C, and 44.5kg of p-chlorobenzotrifluoride was added dropwise. The reaction was controlled at 30°C for 6 hours, and then tetrahydrofuran was recovered, and the residue was cooled to room temperature and placed in 300 kg of ice water (18.5 kg of 35% hydrochloric acid aqueous solution was added to the ice water in advance). After fully hydrolyzed, the layers are separated, and the separated water layer is extracted once with 50L of toluene, and the organic matter obtained is combined with the separated or...
Embodiment 2
[0022] The concrete steps of the preparation method of trifluoromethylbenzyl chloride of the present embodiment comprise:
[0023] A. Add 150kg of pretreated tetrahydrofuran, 7.3kg of dried magnesium particles, 30g of iodine, 1kg of bromoethane and 0.5kg of p-chlorobenzotrifluoride into the reaction kettle, slowly feed nitrogen, stir and heat up to reflux, and keep reflux After 40 minutes, the Grignard reagent was successfully initiated, and then the temperature was controlled at 40°C, and 44.5kg of p-chlorobenzotrifluoride was added dropwise. The temperature was controlled at 20°C for 7 hours, then tetrahydrofuran was recovered, and the residue was cooled to room temperature and placed in 300 kg of ice water (18.5 kg of 35% hydrochloric acid aqueous solution was added to the ice water in advance). After fully hydrolyzed, the layers are separated, and the separated water layer is extracted once with 50L of toluene, and the organic matter obtained is combined with the separated...
Embodiment 3
[0026] The concrete steps of the preparation method of trifluoromethylbenzyl chloride of the present embodiment comprise:
[0027] A. Add 150kg of pretreated tetrahydrofuran, 7.3kg of dried magnesium particles, 30g of iodine, 1kg of bromoethane and 0.5kg of p-chlorobenzotrifluoride into the reaction kettle, slowly feed nitrogen, stir and heat up to reflux, and keep reflux After 20 minutes, the Grignard reagent was successfully initiated, then the temperature was controlled at 60°C, and 44.5kg of p-chlorobenzotrifluoride was added dropwise. The temperature was controlled at 40°C for 5 hours, then tetrahydrofuran was recovered, and the residue was cooled to room temperature and placed in 300 kg of ice water (18.5 kg of 35% hydrochloric acid aqueous solution was added to the ice water in advance). After fully hydrolyzed, the layers are separated, and the separated water layer is extracted once with 50L of toluene, and the organic matter obtained is combined with the separated org...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com