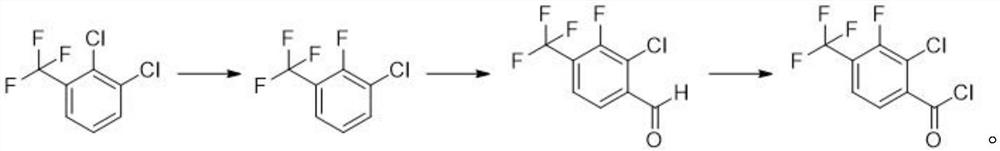
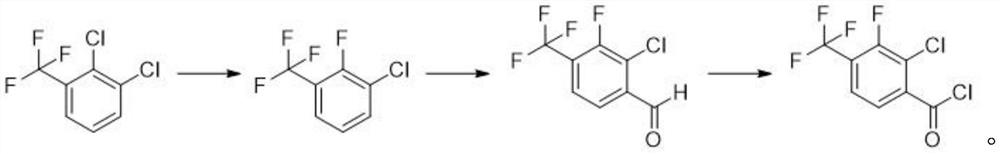
Synthesis method of 2-chloro-3-fluoro-4-trifluoromethyl benzoyl chloride
A technology of trifluoromethylbenzoyl chloride and trifluoromethylbenzaldehyde, which is applied in the field of synthesis of organic chemical intermediates, can solve the problems of low yield and large pollution, achieve less waste, simple reaction operation, and good industrialization foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] a) Preparation of 2-fluoro-3-chlorobenzotrifluoride
[0031] Add 215g of 2,3-dichlorobenzotrifluoride, 73g of potassium fluoride, 7g of triphenylphosphine bromide and 430mL of 1,3-dimethylimidazolidinone into a 1000mL three-necked flask equipped with a rectification column, and stir to raise the temperature Reflux reaction. After 1 hour of reaction, 185 g of a colorless transparent liquid began to evaporate slowly, and the content of GC analysis was 93.9%. The collected crude product was purified by rectification to obtain 160 g of a colorless transparent liquid, namely 2-fluoro-3-chlorotrifluorotoluene. GC content 98.5%, yield 68.3%
[0032] The reaction residue was cooled and filtered with suction, and the filtrate was recovered by distillation under reduced pressure to obtain 410 mL of 1,3-dimethylimidazolidinone.
[0033] b) Preparation of 2-chloro-3-fluoro-4-trifluoromethylbenzaldehyde
[0034] Under the protection of nitrogen, add 500mL of dry tetrahydrofuran ...
Embodiment 2
[0039] a) Preparation of 2-fluoro-3-chlorobenzotrifluoride
[0040] Add 215g of 2,3-dichlorobenzotrifluoride, 100g of potassium fluoride, 3g of triethylamine and 450mL of 1,3-dimethylimidazolidinone into a 1000mL three-necked flask equipped with a rectification column, stir and raise the temperature to reflux for reaction. After 4 hours of reaction, 189 g of a colorless transparent liquid began to slowly distill out, and the content of GC analysis was 95.2%. The collected crude product was purified by rectification to obtain 164 g of a colorless transparent liquid, namely 2-fluoro-3-chlorobenzotrifluoride. GC content 98.7%, yield 70%.
[0041] The reaction residue was cooled and filtered with suction, and the filtrate was recovered by distillation under reduced pressure to obtain 420 mL of 1,3-dimethylimidazolidinone.
[0042] b) Preparation of 2-chloro-3-fluoro-4-trifluoromethylbenzaldehyde
[0043] Under the protection of nitrogen, add 500mL of dry tetrahydrofuran to a 2L...
Embodiment 3
[0049] a) Preparation of 2-fluoro-3-chlorobenzotrifluoride
[0050] Add 215g of 2,3-dichlorobenzotrifluoride, 80g of potassium fluoride, 7g of DBU and 430mL of 1,3-dimethylimidazolidinone into a 1000mL three-necked flask equipped with a rectification column, stir and raise the temperature to reflux for reaction. After 2 hours of reaction, 188 g of a colorless transparent liquid began to slowly evaporate, and the content of GC analysis was 93.2%. The collected crude product was purified by rectification to obtain 165 g of a colorless transparent liquid, namely 2-fluoro-3-chlorobenzotrifluoride. GC content 97.8%, yield 70.4%.
[0051] The reaction residue was cooled and filtered with suction, and the filtrate was recovered by distillation under reduced pressure to obtain 408 mL of 1,3-dimethylimidazolidinone.
[0052] b) Preparation of 2-chloro-3-fluoro-4-trifluoromethylbenzaldehyde
[0053] Under nitrogen protection, add 500mL dry tetrahydrofuran to a 2L three-necked flask, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com