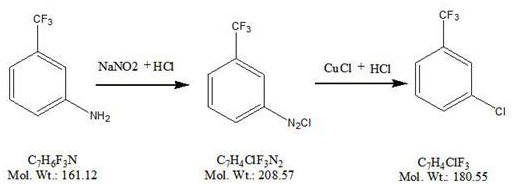
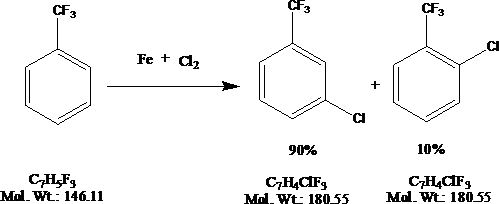
New synthesis process of m-chlorobenzotrifluoride
A technology for m-chlorotrifluorotoluene and chlorotrifluorotoluene is applied in the new synthesis process field of m-chlorotrifluorotoluene, which can solve the problems of difficult separation and purification, large environmental protection pressure, generation of a large amount of waste hydrochloric acid, etc., and achieves abundant sources and high prices. The effect of low, low product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0031] The invention provides a technical scheme: a new synthesis process of m-chlorobenzotrifluoride, comprising the following steps:
[0032] S1. Weigh each component: Weigh 300g of 3,4-dichlorobenzotrifluoride, 300g of cyclohexane, 280g of triethylamine, 280g of water, and 20g of Raney nickel in sequence, and put the above components into 2L in the autoclave;
[0033] S2. Replacement treatment: nitrogen replacement, hydrogen replacement;
[0034] S3. Temperature control: Slowly raise the temperature to 80°C, and control the temperature between 80~85°C;
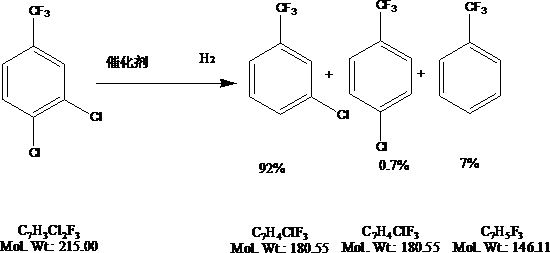
[0035] S4, sampling analysis experimental data: the hydrogen pressure is 2.0MPa, after 4 hours of reaction, sampling analysis, the content is 92% for m-chlorobenzotrifluoride, 0.7% for p-chlorobenzotrifluoride, and 7.3% for benzotrifluoride, control p-Chlorotrifluorotoluene not more than 1%;
[0036] S5, static layering: static layering, the lower layer is an aqueous solution of triethylamine hydrochloride, adjusted to P...
Embodiment example 2
[0040] The invention provides a technical scheme: a new synthesis process of m-chlorobenzotrifluoride, comprising the following steps:
[0041] S1. Weigh each component: Weigh 300g of 3,4-dichlorobenzotrifluoride, 300g of ethanol, 117g of potassium hydroxide, and 10g of ruthenium carbon successively, and put the above-mentioned components into a 2L autoclave successively, wherein , the ruthenium content of ruthenium carbon 10g is 5%, and the dry weight is 40%;
[0042] S2. Replacement treatment: nitrogen replacement, hydrogen replacement;
[0043] S3. Temperature control: Slowly raise the temperature to 60°C, and keep the temperature between 60°C and 63°C;
[0044] S4, sampling analysis experimental data: the hydrogen pressure is 1.0MPa, after 4 hours of reaction, sampling analysis, the content is 94% for m-chlorobenzotrifluoride, 0.5% for p-chlorobenzotrifluoride, and 5.5% for benzotrifluoride, control p-Chlorotrifluorotoluene not more than 1%;
[0045] S5, stand and strat...
Embodiment example 3
[0049]The invention provides a technical scheme: a new synthesis process of m-chlorobenzotrifluoride, comprising the following steps:
[0050] S1. Weigh each component: Weigh 300 g of 3,4-dichlorobenzotrifluoride, 300 g of ethanol, 280 g of triethylamine, and 20 g of Raney nickel in sequence, and put the above components into a 2L autoclave in sequence;
[0051] S2. Replacement treatment: nitrogen replacement, hydrogen replacement;
[0052] S3. Temperature control: Slowly raise the temperature to 70°C, and control the temperature between 70~75°C;
[0053] S4, sampling analysis experimental data: the hydrogen pressure is 1.5MPa, after 3.5 hours of reaction, the sampling analysis shows that the content is 88% for m-chlorobenzotrifluoride, 0.8% for p-chlorobenzotrifluoride, and 11.2% for benzotrifluoride. p-Chlorotrifluorotoluene not more than 1%;
[0054] S5, standing and stratifying: standing and filtering to remove the catalyst, ethanol is obtained through desolventization, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com