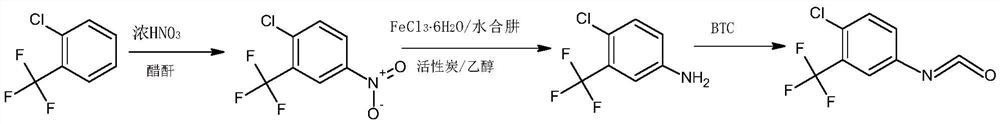
The synthetic method of 4-chloro-3-(trifluoromethyl)benzene isocyanate
A technology of phenyl isocyanate and trifluoromethyl is applied in the field of synthesis of 4-chloro-3-phenyl isocyanate, which can solve the problems of difficult handling of iron sludge waste, easy generation of isomer impurities, hidden danger of hydrogen, etc., and reduce environmental protection The effect of stress, low risk, high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
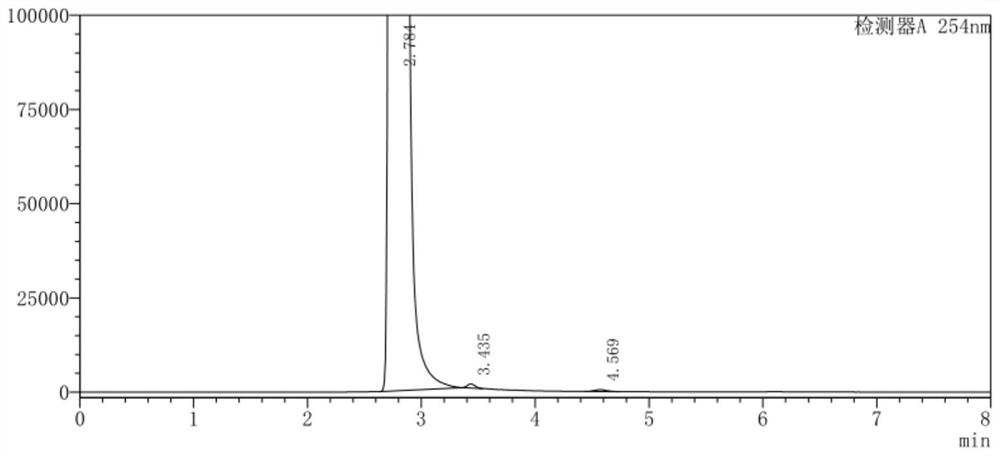
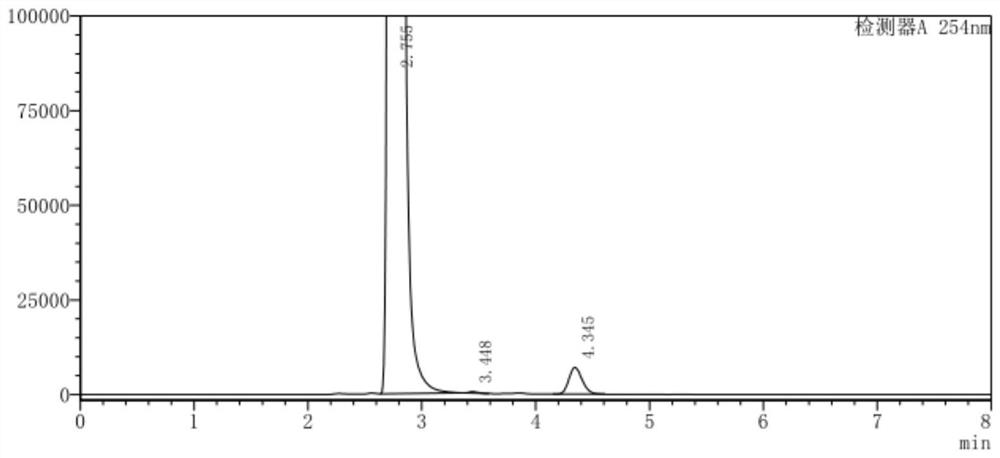
Image
Examples
Embodiment 1
[0045] (1) In a 500ml three-necked flask, add 100g of o-chlorobenzotrifluoride and 200g of acetic anhydride and mix well, control the temperature at 10-12°C, add 65g of concentrated nitric acid dropwise, keep stirring for 3 hours after dropping, add 5% sodium hydroxide aqueous solution Adjust the pH to 8.0, separate the layers after washing, and collect 120.7 g of the organic phase;
[0046] (2) the organic phase obtained in step (1), 1g activated carbon, 7.5gFeCl 3 ·6H 2 O was added to 200 g of ethanol, mixed uniformly and then heated to reflux, and 86.5 g of hydrazine hydrate solution with a mass fraction of 80% was added dropwise, and the addition was completed within 3 hours. After the dropwise addition was completed, filter while hot, distill the filtrate to remove ethanol under normal pressure, add 1,2-dichloroethane for extraction and layering, and collect the organic phase;
[0047] (3) Add 65.7g of triphosgene and 1g of DMAP into 1,2-dichloroethane, stir until disso...
Embodiment 2
[0053] (1) In a 500ml three-neck flask, add 100g of o-chlorobenzotrifluoride and 220g of acetic anhydride and mix well, add 68g of concentrated nitric acid dropwise at a controlled temperature of 10-12°C, keep stirring for 3.5h after dropping, add 5% sodium hydroxide The pH of the aqueous solution was adjusted to 7.8, the layers were separated after washing, and 123.1 g of the organic phase was collected;
[0054] (2) the organic phase obtained in step (1), 1g activated carbon, 9.6gFeCl 3 ·6H 2 O was added to 200 g of ethanol, mixed uniformly and then heated to reflux, and 88.2 g of hydrazine hydrate solution with a mass fraction of 80% was added dropwise, and the addition was completed within 3 hours. After the dropwise addition is completed, filter while it is hot, evaporate the ethanol from the filtrate under normal pressure, add dioxane for extraction and layering, and collect the organic phase;
[0055] (3) Add 65.7g of triphosgene and 1g of pyridine into dioxane, stir ...
Embodiment 3
[0058] (1) In a 500ml three-necked flask, add 100g of o-chlorobenzotrifluoride and 210g of acetic anhydride and mix well, add 69g of concentrated nitric acid dropwise at a controlled temperature of 12-14°C, keep stirring for 4 hours after dropping, add 5% sodium hydroxide aqueous solution Adjust the pH to 7.5, separate the layers after washing, and collect 119.7 g of the organic phase;
[0059] (2) the organic phase obtained in step (1), 1g activated carbon, 8.4gFeCl 3 ·6H 2 O was added to 200 g of ethanol, mixed uniformly and then heated to reflux, and 83.9 g of hydrazine hydrate solution with a mass fraction of 80% was added dropwise, and the addition was completed within 3.5 hours. After the dropwise addition was completed, filter while it was hot, evaporate the ethanol from the filtrate under normal pressure, add chloroform for extraction and layering, and collect the organic phase;
[0060] (3) Add 65.7g of triphosgene and 1g of DMF into chloroform, stir until dissolved...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com