Preparation method of 2-fluoro-4-cyano trifluorotoluene
A technology of cyanobenzotrifluoride and chlorobenzotrifluoride, which is applied in the field of preparation of 2-fluoro-4-cyanobenzotrifluoride, can solve the problems of expensive raw materials, high raw material prices, and complicated processes, and achieve the goal of reducing impurities The effect of generating, improving product purity, and improving conversion yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
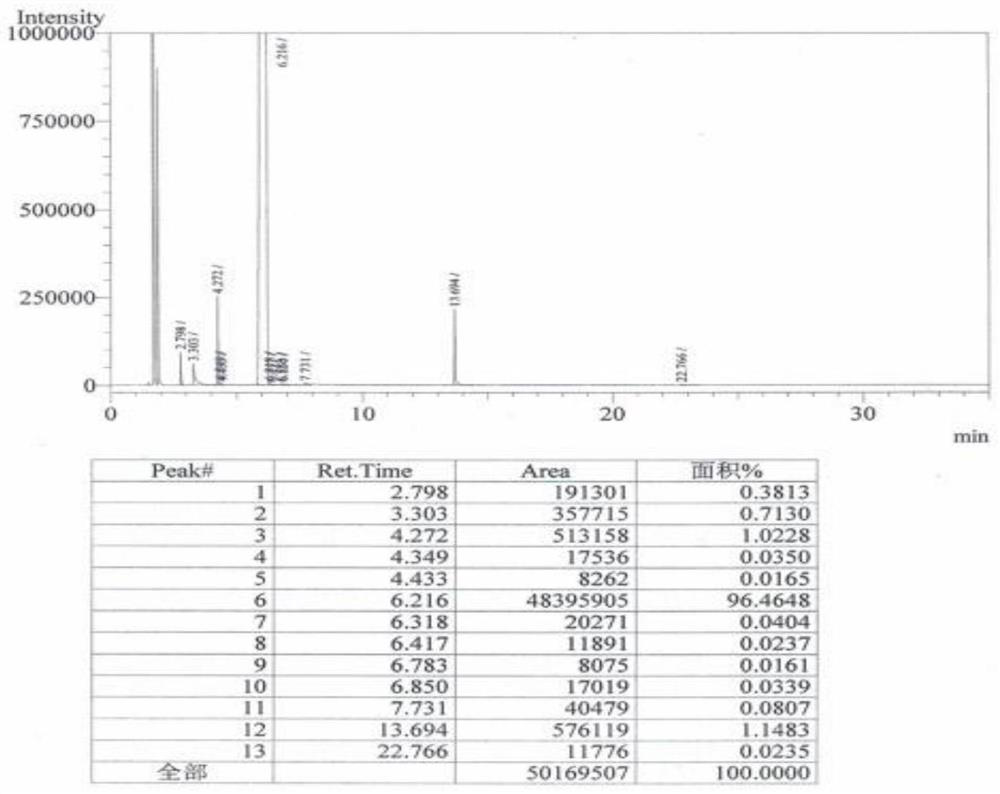
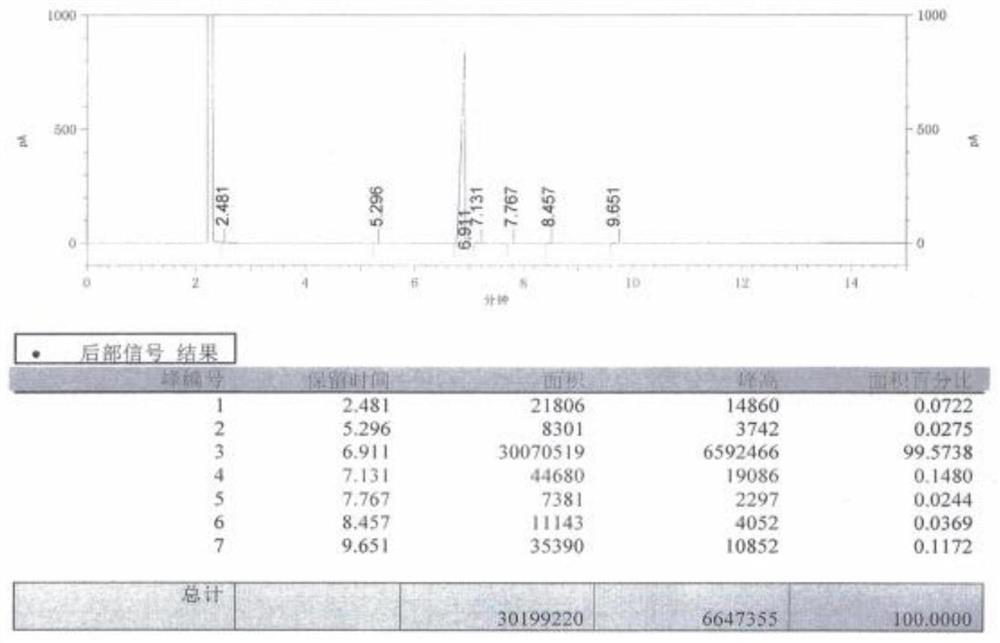
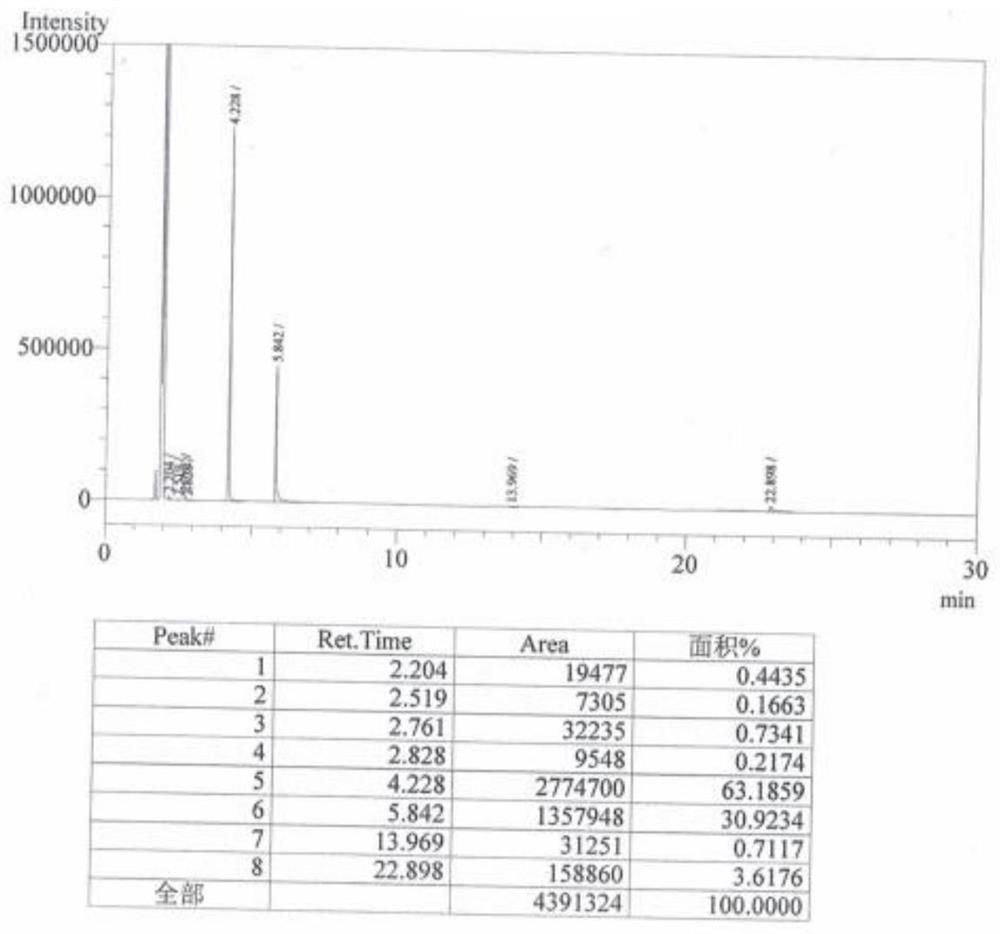
Image
Examples
Embodiment 1
[0026] A preparation method of 2-fluoro-4-cyanobenzotrifluoride, prepared according to the following method: the reaction chemical formula is as follows
[0027]
[0028] The preparation method is as follows:
[0029] (1) Preparation of activated zinc powder (used for the preparation of tetrakis(triphenylphosphine) nickel)
[0030] Add 10L methyl ethyl ketone and 1kg acetic acid to a 20L four-necked bottle, start stirring, add 10kg zinc powder, use a heating mantle to raise the temperature of the system to reflux to 75°C, cool down to T<40°C after 2 hours, and pour the activated zinc powder system into Put it into the system ① in a 50L barrel for use.
[0031] (2) Preparation of four (triphenylphosphine) nickel (main reaction catalyst)
[0032] 1. Add 7kg of anhydrous nickel chloride, 62.5kg of triphenylphosphine, and 130kg of DMF into a dry and clean 200L oil bath kettle, use the jacket of the oil bath kettle to raise the temperature of the system to T=168~172℃, and stea...
Embodiment 2
[0041] The difference from Example 1 is: add 7kg of anhydrous nickel chloride, 52.5kg of triphenylphosphine, and 150kg of DMF to a dry and clean 200L oil bath kettle, and use the jacket of the oil bath kettle to raise the temperature of the system to T=168~ At 172°C, 80kg of DMF can be evaporated in about 2 hours, then nitrogen gas is passed into the oil bath kettle, and the temperature is lowered to T=60-70°C under the protection of nitrogen gas, and the system ② is obtained for use.
Embodiment 3
[0043] The difference from Example 1 is: add 7kg of anhydrous nickel chloride, 62.5kg of triphenylphosphine, and 130kg of DMF to a dry and clean 200L enamel kettle, and use the jacket of the enamel kettle to raise the temperature of the system to T=120~130℃ , about 2 hours to steam out 80kg of DMF, and then pass nitrogen into the oil-bath kettle, nitrogen protection to lower the temperature to T = 60 ~ 70 ℃, the system ② is ready to use. Embodiment Four
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com