Novel nitration process of 3,5-binitro-4-chlorobenzotrifluoride
A technology of nitrotrifluorotoluene and chlorotrifluorotoluene, which is applied in 3 fields, can solve the problems of low reaction temperature, long reaction period, volatilization of nitric acid, etc., and achieves the effects of less corrosion of equipment, reduction and elimination of pollution, and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
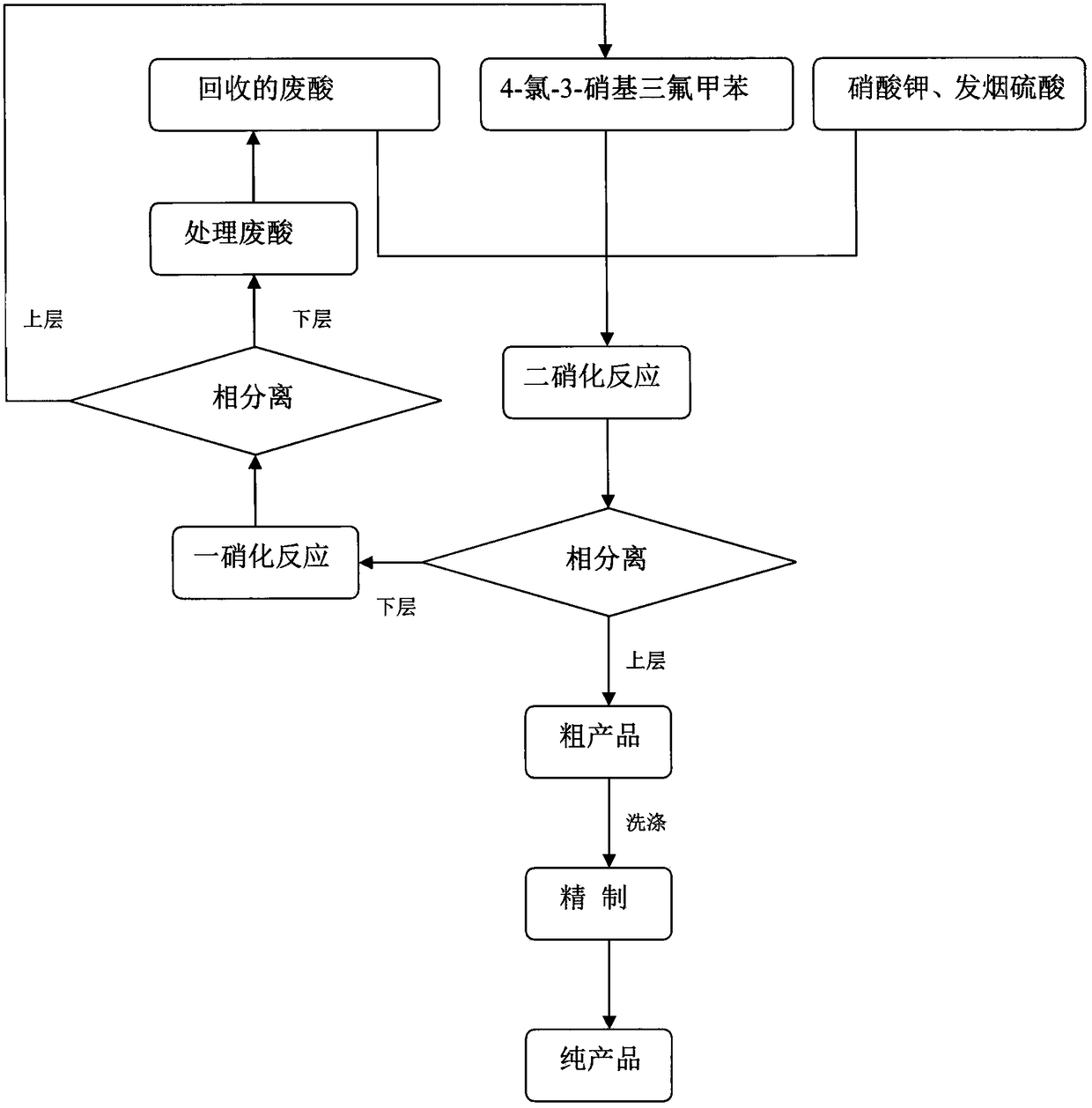
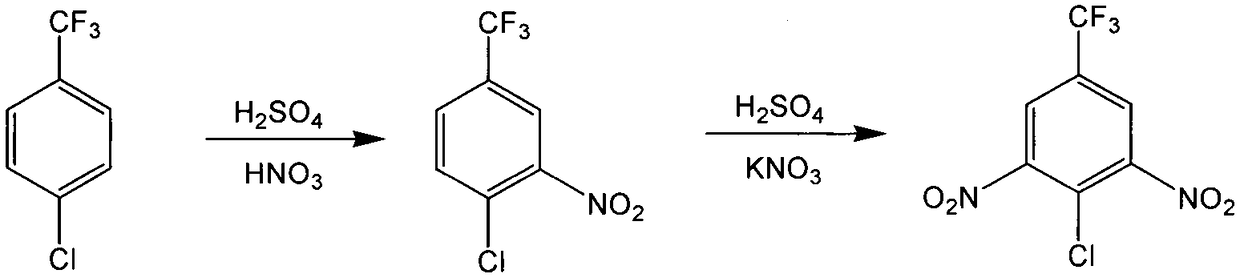
[0019] In a 500mL four-necked flask, 35g of p-chlorotrifluorotoluene, 55g of 68% nitric acid, 10g of N,N-dimethyl-N-dodecyl-N-sulfonic acid propyl ammonium hydrogen sulfate were added successively, and the mixture was added to 50 Mix and stir at ℃ for 4 hours, cool and stand for phase separation, the upper layer of crude product is washed with water and neutralized by vacuum drying to obtain pure product 4-chloro-3-nitrotrifluorotoluene, which is used for the next step of dinitration reaction; the lower layer of waste acid After cyclohexane extraction and drying, it can be recycled for dinitration process.
Embodiment 2
[0021] In a 500mL four-necked flask, 10g of mononitrated product 4-chloro-3-nitrotrifluorotoluene prepared in Example 1, 10g of potassium nitrate, 20g of 30% oleum, 10 g of the treated waste acid was mixed and stirred at 80°C for 5 hours, left to stand for phase separation, and the upper crude product was washed with water, neutralized, purified, and dried in vacuo to obtain pure dinitration product 4-chloro-3,5-dinitrotrifluoro Toluene, the yield is 82%; the semi-waste acid in the lower layer is directly used in the mononitration reaction process.
Embodiment 3
[0023] In a 500mL four-necked flask, 10g of p-chlorotrifluorotoluene and 30g of semi-waste acid produced in Example 2 were added successively, mixed and stirred at 50°C for 3 hours, cooled and left to stand for phase separation, and the upper layer of the crude product was washed with water and neutralized by vacuum. The pure product 4-chloro-3-nitrotrifluorotoluene is obtained by drying, which is used for the next step of dinitration reaction; the lower layer waste acid is extracted with cyclohexane, dried, and filtered to remove the inorganic salt precipitation, which can be recycled for the dinitration process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com