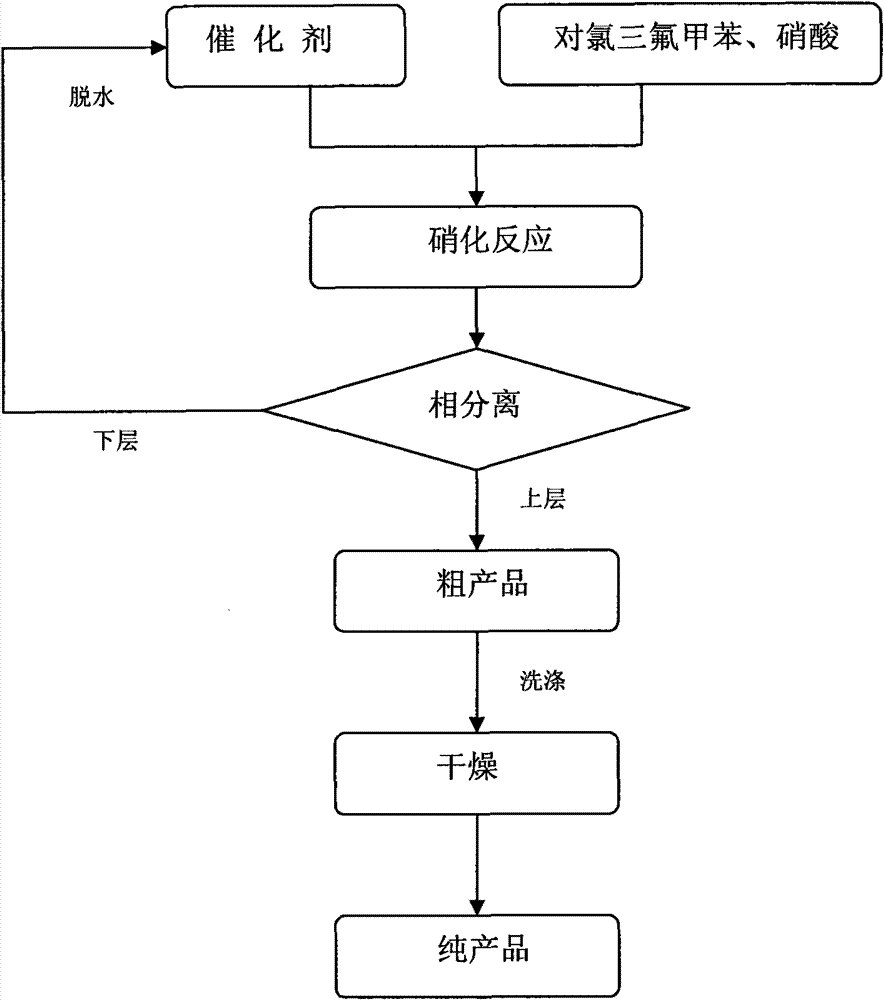
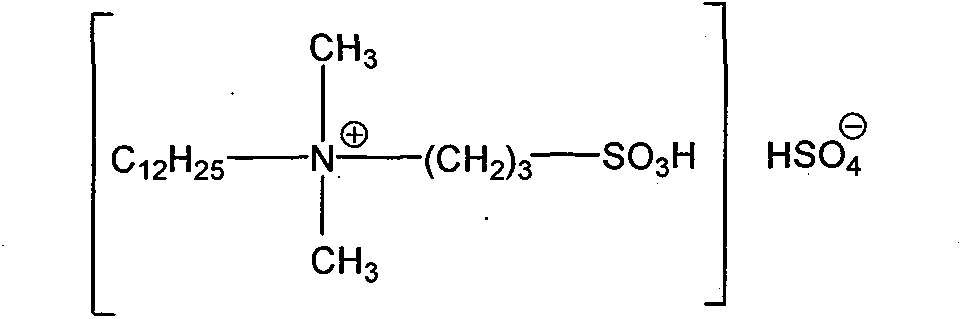
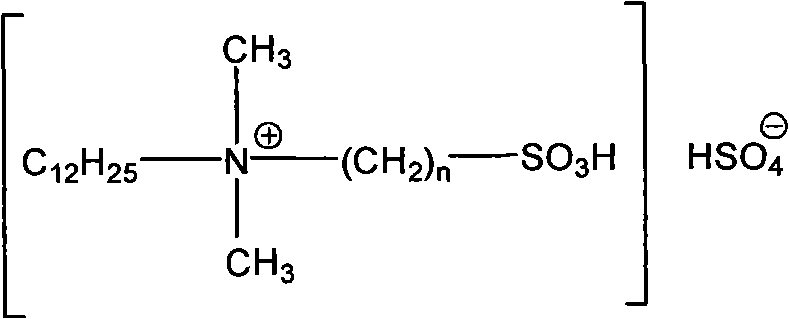
Clean nitration reaction of p-chlorobenzotrifluoride under catalysis of degradable functional ionic liquid
A technology of p-chlorotrifluorotoluene and ionic liquid, applied in physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, organic chemistry, etc., can solve the difficulty of increasing post-processing, product separation, imidazole ionic liquids are expensive, cannot meet environmental and economic problems, and achieve the effects of good industrial application prospects, environmental friendliness, and convenient preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 25mL round bottom flask, add 10mmol (1.81g) of p-chlorobenzotrifluoride, 30mmol (2.8g) of 68% nitric acid, and 1.5mmol (0.65g) of catalyst in sequence, mix and stir for 10 hours at 100°C, cool, and statically The phases were separated, the crude product in the upper layer was washed with water, neutralized and dried in vacuum to obtain the pure product 4-chloro-3-nitrobenzotrifluoride with a yield of 80%.
Embodiment 2
[0024] In a 25mL round bottom flask, add 10mmol (1.81g) of p-chlorobenzotrifluoride, 10mmol (0.63g) of 100% nitric acid, and 1mmol (0.43g) of catalyst in turn, mix and stir at 30°C for 8 hours, cool and stand The phases were separated, the crude product in the upper layer was washed with water, neutralized and dried in vacuum to obtain the pure product 4-chloro-3-nitrobenzotrifluoride with a yield of 81%.
Embodiment 3
[0026] In a 25mL round bottom flask, add 10mmol (1.81g) of p-chlorobenzotrifluoride, 20mmol (1.4g) of 90% nitric acid, and 0.5mmol (0.22g) of catalyst in turn, mix and stir for 5 hours at 80°C, cool and statically The phases were separated, the crude product in the upper layer was washed with water, neutralized and dried in vacuum to obtain the pure product 4-chloro-3-nitrobenzotrifluoride with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com