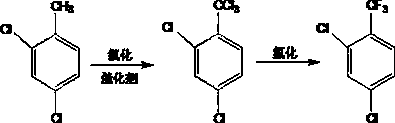
2,4-dichlorobenzotrifluoride preparation method
A technology of dichlorobenzotrifluoride and dichlorotrichlorotoluene, which is applied in the field of preparation of pesticide chemical intermediate products, can solve the problems of high cost, principle, and route without breaking through the step-by-step reaction method, and achieve low equipment corrosion and social Significant benefit and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Chlorination: put 800g of 2,4-dichlorotoluene into a 3L reactor, raise the temperature to 115°C, add 40g of catalyst, and start to feed the flow at 40-80 m 3 / h of dry chlorine, the temperature is controlled at 115-117°C, and the reaction time is 24 hours. When the content of 2,4-dichlorodichlorotoluene is less than 0.5%, stop feeding chlorine gas, and change into dry air to discharge unreacted chlorine gas, hydrogen chloride gas and gas impurities produced by the reaction to obtain 2,4-dichlorotrichloride Chlorotoluene.
[0032] (2) Fluorination: Press 4 moles of 2,4-dichlorobenzotrichloride and 12 moles of anhydrous hydrogen fluoride into a 3L reactor, and check the opening status of each valve. Turn on the stirring, slowly feed steam into the jacket, and when the pressure in the kettle reaches 1.5MPa, open the tail gas discharge valve to exhaust, continue to heat up until the temperature in the kettle is 110°C, and control the pressure in the kettle at 1.5-1.7MP...
Embodiment 2
[0036] (1) Chlorination: Put 800g of 2,4-dichlorotoluene into a 3L reactor, raise the temperature to 115°C, add 80g of catalyst, and start to feed the flow at 40-80 m 3 / h of dry chlorine gas, the temperature is controlled at 120-125°C, and the reaction time is 22 hours. When the content of 2,4-dichlorodichlorotoluene is less than 0.5%, stop feeding chlorine gas, and change into dry air to discharge unreacted chlorine gas, hydrogen chloride gas and gas impurities produced by the reaction to obtain 2,4-dichlorotrichloride Chlorotoluene.
[0037] (2) Fluorination: Press 4 moles of 2,4-dichlorobenzotrichloride and 14 moles of anhydrous hydrogen fluoride into a 3L reactor, and check the opening status of each valve. Turn on stirring, slowly feed steam into the jacket, and when the pressure in the kettle reaches 1.5MPa, open the exhaust valve to exhaust, continue to heat up until the temperature in the kettle is 115°C, and control the pressure in the kettle to 2.2-2.5MPa at the sa...
Embodiment 3
[0041] (1) Chlorination: put 800g of 2,4-dichlorotoluene into a 3L reactor, raise the temperature to 115°C, add 60g of catalyst, and start to feed the flow at 40-80 m 3 / h of dry chlorine gas, the temperature is controlled at 115-120°C, and the reaction time is 20 hours. When the content of 2,4-dichlorodichlorotoluene is less than 0.5%, stop feeding chlorine gas, and change into dry air to discharge unreacted chlorine gas, hydrogen chloride gas and gas impurities produced by the reaction to obtain 2,4-dichlorotrichloride Chlorotoluene.
[0042] (2) Fluorination: Press 4 moles of 2,4-dichlorotrichlorotoluene and 13 moles of anhydrous hydrogen fluoride into a 3L reactor, and check the opening status of each valve. Turn on stirring, slowly feed steam into the jacket, and when the pressure in the kettle reaches 1.5MPa, open the exhaust valve to exhaust, and continue to heat up until the temperature in the kettle is 110°C, and at the same time control the pressure in the kettle to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com