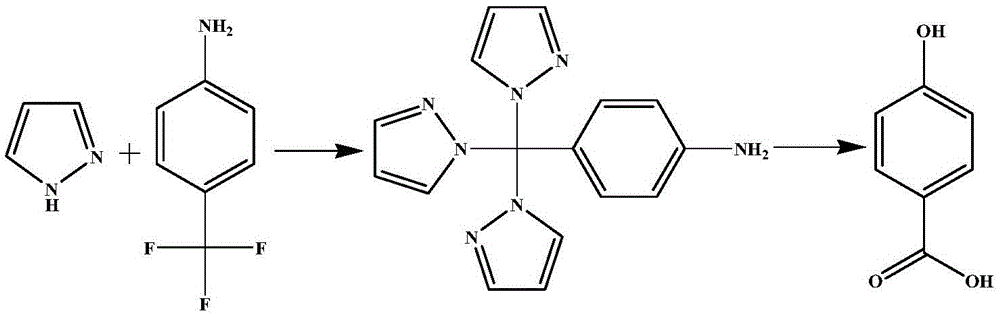
Preparation method for p-hydroxybenzoic acid
A technology of p-hydroxybenzoic acid and trifluoromethylaniline, which is applied in the field of preparation of p-hydroxybenzoic acid, can solve the problems of low yield, high cost, long reaction time, etc., and achieves simple post-treatment, short synthesis route and fast reaction time. short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
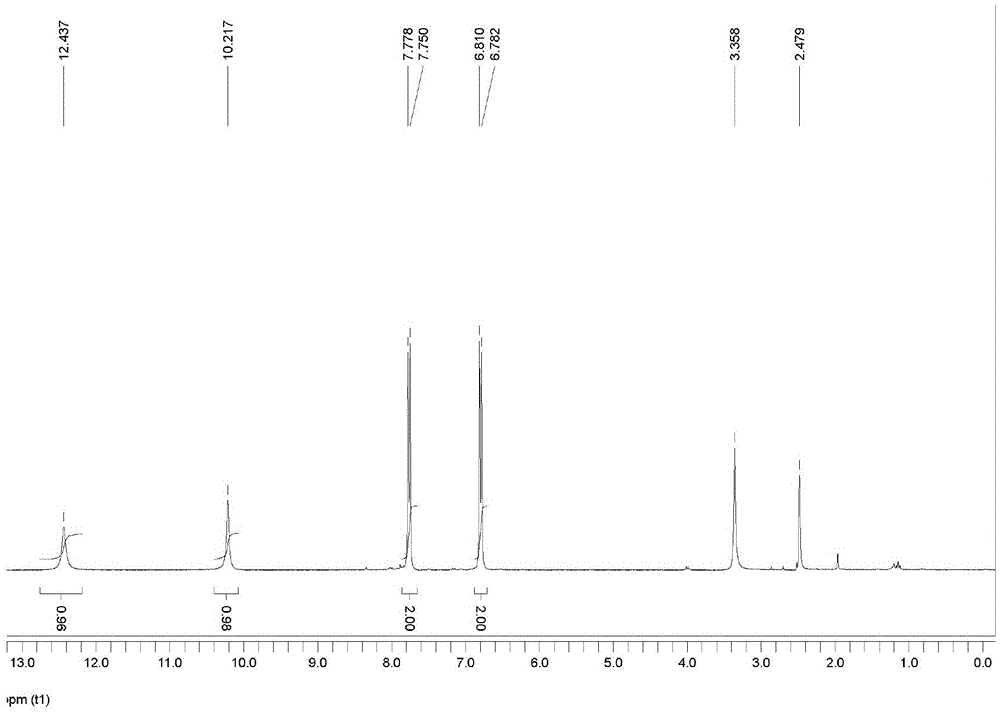
[0017] Add 100mL dimethyl sulfoxide into a 500mL two-necked flask, add 20.5g pyrazole, 16g p-trifluoromethylaniline, and 17g potassium hydroxide in sequence, stir and dissolve, heat to reflux for 1 hour, and cool to room temperature. Add 200mL of water to dissolve, add dichloromethane and extract three times, 200mL each time. The organic phase was dried with anhydrous magnesium sulfate and separated by column chromatography to obtain 24.3 g of 4-tripyrazolylmethylaniline as a light yellow solid with a yield of 80.4%.
[0018] Add 18.3g of 4-tripyrazolylmethylaniline and 60mL of water into a three-necked flask equipped with a reflux condenser, slowly add 15mL of concentrated sulfuric acid and stir within 15 minutes, and add 23.3% 19.6 g of sodium nitrite aqueous solution was stirred for 15 minutes, and 16.5 mL of 15.5% sulfuric acid solution was added dropwise within 15 minutes. The temperature was raised to 50°C for 1 h, cooled naturally, and ethyl acetate was added for separ...
Embodiment 2
[0020] Add 20mL of dimethyl sulfoxide into a 100mL two-necked flask, add 4.1g of pyrazole, 3.2g of p-trifluoromethylaniline and 3.4g of potassium hydroxide in sequence, stir and dissolve, heat to reflux for 1h, and cool to room temperature. Add 40mL of water to dissolve, add dichloromethane and extract three times, 40mL each time. The organic phase was dried with anhydrous magnesium sulfate and separated by column chromatography to obtain 4.98 g of light yellow solid 4-tripyrazolylmethylaniline with a yield of 81.5%.
[0021] Add 3.05g of 4-tripyrazolylmethylaniline and 10mL of water into a three-necked flask equipped with a reflux condenser, slowly add 2.5mL of concentrated sulfuric acid dropwise within 5 minutes and stir, then add 23.3% sulfuric acid in an ice bath for 5 minutes. Sodium nitrate aqueous solution 1.3g, stirred for 15min. 1.1 mL of 15.5% sulfuric acid solution was added dropwise over 5 minutes. Raise the temperature to 100°C for 15 minutes, cool naturally, ad...
Embodiment 3
[0023] Add 10 mL of dimethyl sulfoxide to a 50 mL two-necked flask, add 2.05 g of pyrazole, 1.6 g of p-trifluoromethylaniline, and 1.7 g of potassium hydroxide in sequence, stir and dissolve, heat to reflux for 1 h, and cool to room temperature. Add 20mL of water to dissolve, add dichloromethane and extract three times, 20mL each time. The organic phase was dried with anhydrous magnesium sulfate, and separated by column chromatography to obtain 2.35 g of light yellow solid 4-tripyrazolylmethylaniline with a yield of 77.7%.
[0024] Add 1g of 4-tripyrazolylmethylaniline and 4mL of water into a three-necked flask equipped with a reflux condenser, slowly add 2mL of concentrated sulfuric acid dropwise within 5 minutes and stir, then slowly add 23.3% sulfuric acid in an ice bath for 5 minutes. 0.5 g of sodium nitrate aqueous solution was stirred for 15 min, and 0.4 mL of 15.5% sulfuric acid solution was added dropwise within 5 min. Raise the temperature to 30°C for 2 hours, cool n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com