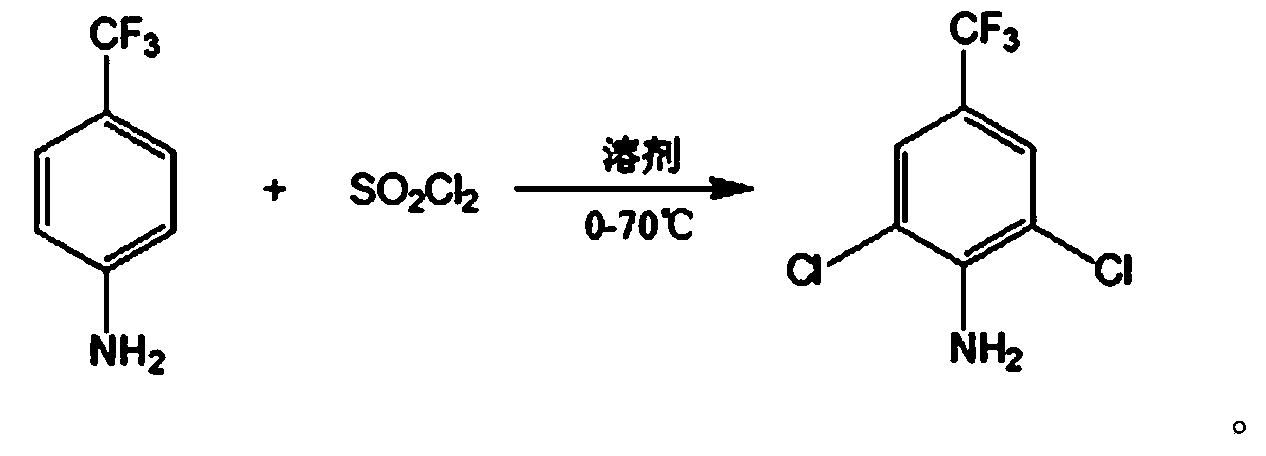
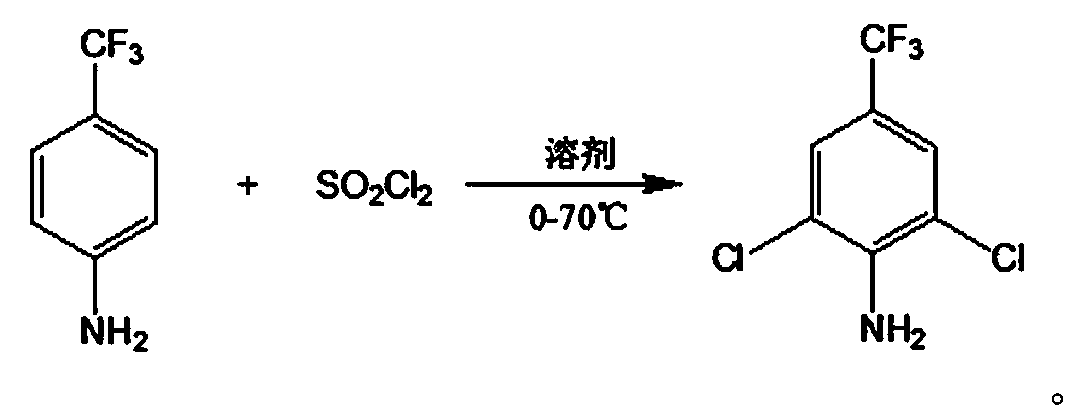
Method for preparing 2,6-dichloro-4-trifluoromethyl phenylamine
A technology of trifluoromethylaniline and dichloride, which is applied in the preparation of amino compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of hidden production safety hazards and high equipment requirements, and achieves low production cost and short reaction time. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 200g of p-trifluoromethylaniline and 480g of dichloroethane to a 3L reaction kettle, control the temperature to 10°C, add dropwise a solution of 503g of sulfuryl chloride and 190g of dichloroethane under stirring within 3 hours, after the dropwise addition is completed, in Continue to react at 50°C for 2 hours. After the reaction is completed, cool down, wash with water, and wash with saturated sodium carbonate aqueous solution to remove the inorganic salts. After the organic phase is concentrated to remove the solvent, rectify to obtain 2,6-dichloro-4-trifluoromethylaniline 144g.
Embodiment 2
[0025] Add 200g of p-trifluoromethylaniline and 720g of dichloroethane to a 3L reactor, control the temperature to 25°C, add dropwise a mixed solution of 496g of sulfonyl chloride and 190g of dichloroethane under stirring within 3 hours, after the dropwise addition is completed, in Continue to react at 60°C for 4 hours, lower the temperature, wash with water and saturated aqueous sodium carbonate solution to remove inorganic salts, concentrate the organic phase to remove the solvent, and rectify to obtain 160 g of 2,6-dichloro-4-trifluoromethylaniline.
Embodiment 3
[0027] Add 200g of p-trifluoromethylaniline and 700g of N,N-dimethylformamide to a 3L reactor, control the temperature to 40°C, add a mixed solution of 485g of sulfuryl chloride and 190g of dichloroethane dropwise with stirring within 3 hours, dropwise After the addition, continue the reaction at 70°C for 1 hour, lower the temperature, wash with water, and wash with saturated sodium carbonate aqueous solution to remove the inorganic salts, concentrate the organic phase to remove the solvent, and rectify to obtain 2,6-dichloro-4-trifluoromethyl Aniline 120g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com