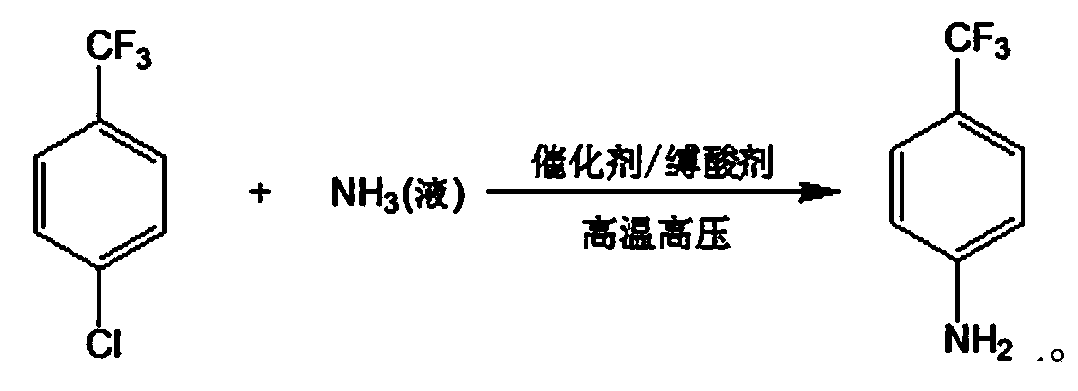
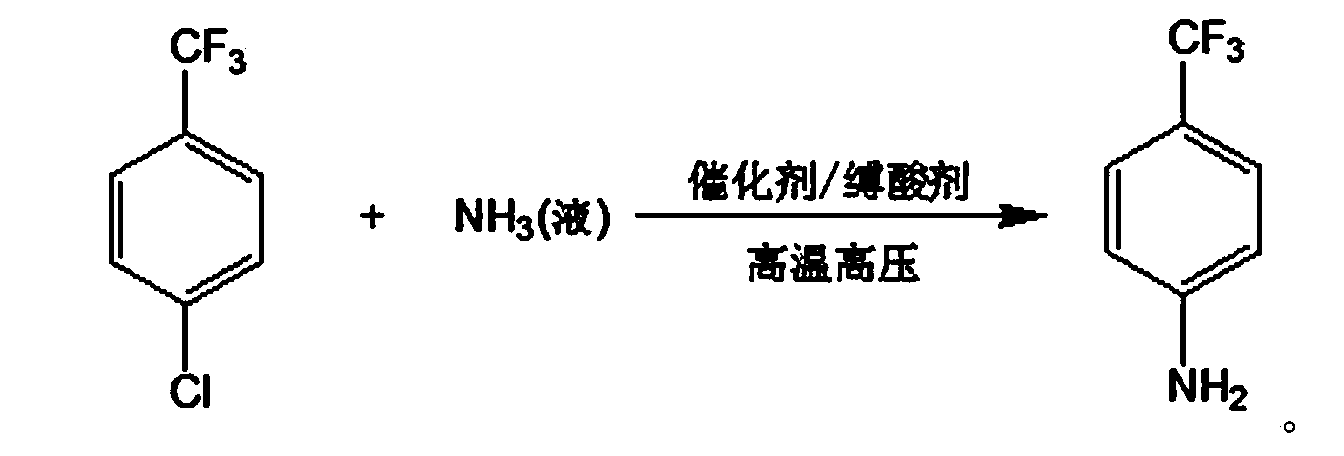
Method for preparing p-trifluoromethylaniline by performing high pressure ammonolysis
A technology of trifluoromethylaniline and p-chlorobenzotrifluoride, which is applied in the field of high-pressure ammonolysis to prepare p-trifluoromethylaniline, can solve the problems of high cost of trifluoromethyl reagents, low conversion rate, and difficult post-processing, etc. Achieve the effects of avoiding side reactions of hydrolysis, convenient recycling, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] To the 3L autoclave, add 300g p-chlorotrifluorotoluene, 25g cuprous chloride, 10g copper powder, 90g sodium carbonate, 900g polyethylene glycol 300, seal the autoclave, and after three times of nitrogen replacement, feed into the autoclave. 150g of liquid ammonia was then heated to 200°C under a pressure of 10MPa, reacted at this temperature for 10h, then cooled to room temperature and filtered to remove insoluble solids, and the obtained liquid was rectified under reduced pressure to obtain 150g of unreacted raw material p-chlorotrifluorotoluene, And 102g of product p-trifluoromethylaniline. Unreacted raw materials can be recovered and recycled.
Embodiment 2
[0029] To the 3L autoclave, add 300g p-chlorotrifluorotoluene, 25g cuprous chloride, 10g copper powder, 90g sodium bicarbonate, 900g ethanol, seal the autoclave, and after three times of nitrogen replacement, feed 150g liquid ammonia into the autoclave , and then heated to 200°C under a pressure of 10MPa, reacted at this temperature for 12h, then cooled to room temperature and filtered to remove insoluble solids. The obtained liquid was rectified under reduced pressure to obtain 158g of p-chlorotrifluorotoluene and 92g of p-trifluoromethylaniline.
Embodiment 3
[0031] Into the 3L autoclave, add 300g p-chlorotrifluorotoluene, 2.5g cuprous chloride, 0.5g copper powder, 60g sodium hydroxide, 600g methanol, seal the autoclave, and after three times of nitrogen replacement, feed 240g into the autoclave The liquid ammonia was heated to 210°C and the pressure was 11MPa, and the reaction was carried out at this temperature for 12h, then cooled to room temperature and filtered to remove insoluble solids. The obtained liquid was rectified under reduced pressure to obtain 155g of p-chlorotrifluorotoluene, 95g of p-trifluoromethyl aniline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com