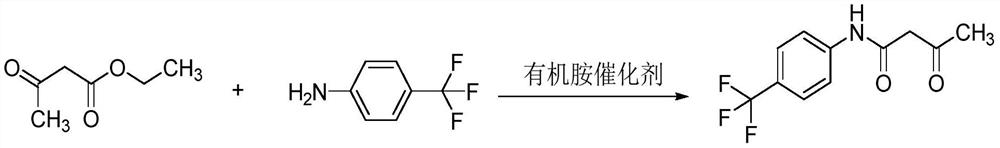
Preparation method of 3-oxo-N-(4-trifluoromethylphenyl) butylamide
A technology of trifluoromethylphenyl and trifluoromethylaniline, which is applied in the field of preparation of 3-oxo-N-butyramide, can solve the problems of unfavorable industrial production, high cost, and large solvent toxicity coefficient, and achieve cost Low, less by-products, the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
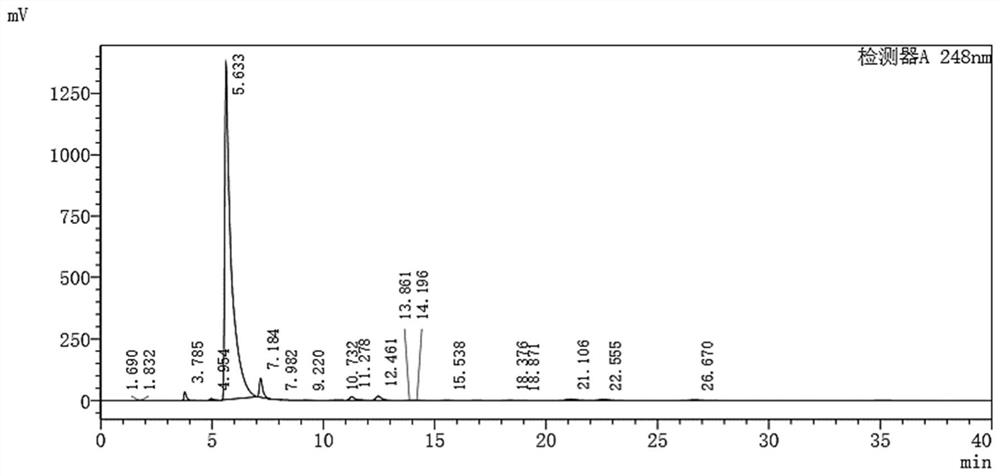
[0022] Put 120g of ethyl acetoacetate into a 1000ml dry and clean four-necked bottle, start the mechanical stirring device, then add 300g of p-trifluoromethylaniline and 0.27g of sodium methoxide successively, and vacuumize N 2 After protection, slowly heat to an internal temperature of 80° C., and keep the reaction for 1 hour. Then, the temperature was raised at a rate of 10°C per hour, and the temperature was raised to 100°C in 2.5 hours, and the temperature was maintained for 20 hours, and the distilled ethanol was received. Stop heating and cool down naturally, then slowly heat and pressurize to concentrate and recover the solvent. The residue is the product, and the purity of the 3-oxygen-N-(4-trifluoromethylphenyl) butyramide obtained in the present embodiment is detected by HPLC, see figure 1 , calculated according to the peak area normalization method, the purity of the 3-oxo-N-(4-trifluoromethylphenyl)butyramide prepared in this example is 94.6%.
Embodiment 2
[0024] Put 120g of ethyl acetoacetate into a 1000ml dry and clean four-neck bottle, start the mechanical stirring device, then add 575g of p-trifluoromethylaniline and 0.27g of tri-n-butylamine in turn, vacuumize N 2 After protection, slowly heat to an internal temperature of 80° C., and keep the reaction for 1 hour. Then the temperature was raised at a rate of 10° C. per hour, and the temperature was raised to an internal temperature of 120° C. in 2.5 hours, and the reaction was kept for 15 hours, and the distilled ethanol was received. Stop heating and cool down naturally, then slowly heat and pressurize to concentrate and recover the solvent. The residue is the product with a purity of 98.2%.
Embodiment 3
[0026] Put 120g of ethyl acetoacetate into a 1000ml dry and clean four-neck bottle, start the mechanical stirring device, then add 100g of p-trifluoromethylaniline and 0.27g of N-methylpyrrolidone in sequence, and vacuumize N 2 After protection, slowly heat to an internal temperature of 80° C., and keep the reaction for 1 hour. Then the temperature was raised at a rate of 10° C. per hour, and the temperature was raised to an internal temperature of 140° C. in 2.5 hours, and the temperature was maintained for 10 hours, and the distilled ethanol was received. After the heat preservation is over, the ethanol in the receiving tank is recovered. Stop heating and cool down naturally, then slowly heat and pressurize to concentrate and recover the solvent. The residue is the product with a purity of 96.09%.
[0027] Visible by above-mentioned implementation process, the present invention has abandoned special reaction solvent, by adding excessive reaction raw material is solvent, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com