Method for preparing carbonyl fluoride from p-trichloromethylphenyl isocyanate
A technology of trichloromethylbenzene isocyanate and carbonyl fluoride, which can be used in the preparation of chlorides, chemical instruments and methods, preparation of amino-substituted functional groups, etc., can solve problems such as difficult reactions, and achieve good safety and high reaction yield. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
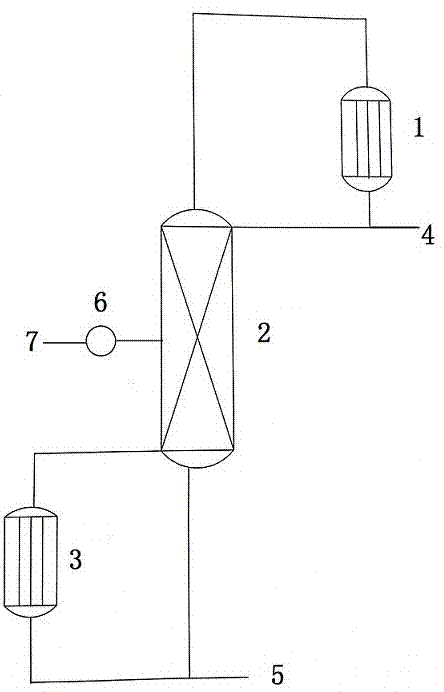
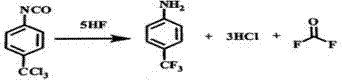
[0038] The following will take p-trichloromethylphenylisocyanate as an example to further describe the present invention in detail, but the specific examples described below are only examples of the present invention and do not constitute any limitation to the present invention. All or part of the surface of the reaction device in the present invention is made of corrosion-resistant materials such as carbonyl fluoride, hydrogen fluoride, hydrogen chloride and hydrofluoric acid. The overall response is as follows:
[0039]
[0040] After the nitrogen leak test, vacuum the alloy autoclave, add 300Kg of p-trichloromethylbenzene isocyanate and 761Kg of anhydrous hydrogen fluoride under negative pressure, start stirring, control the temperature at 0°C, and react for 5 hours, then start to release the pressure slowly. to normal pressure.
[0041] The ice bath was removed, and the temperature of the oil bath was raised to 80° C., the pressure was controlled at 1.0 MPa, and the te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com