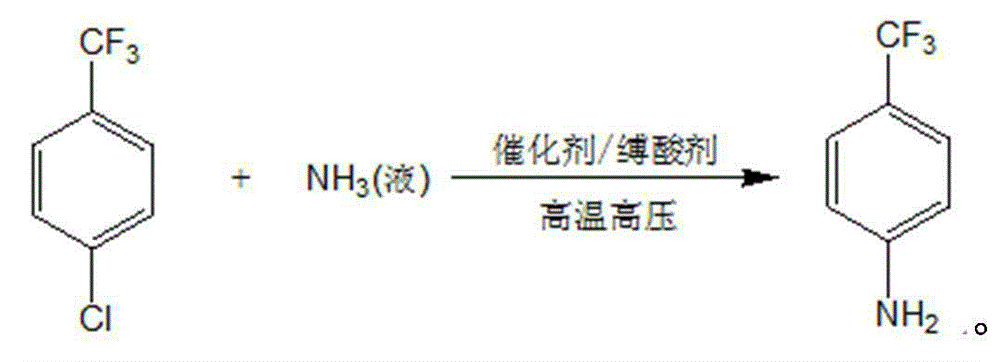
Method for preparing p-trifluoromethylaniline by performing high pressure ammonolysis
A technology of trifluoromethylaniline and p-chlorobenzotrifluoride, which is applied in the field of high-pressure ammonia solution to prepare p-trifluoromethylaniline, can solve the problems of low conversion rate, difficult post-processing, and high cost of trifluoromethyl reagents, and achieves Recycling is convenient, avoiding side reactions of hydrolysis, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 300g of p-chlorobenzotrifluoride, 25g of cuprous chloride, 10g of copper powder, 90g of sodium carbonate, 900g of polyethylene glycol 300 to a 3L autoclave, seal the autoclave, blow nitrogen for three times, then pass into the autoclave 150g of liquid ammonia, then heated to 200°C, pressure of 10MPa, reacted at this temperature for 10h, then cooled to room temperature, filtered to remove insoluble solids, the resulting liquid was rectified under reduced pressure to obtain 150g of unreacted raw material p-chlorobenzotrifluoride, And 102g of product p-trifluoromethyl aniline. Unreacted raw materials can be recovered and recycled.
Embodiment 2
[0029] Add 300g of p-chlorobenzotrifluoride, 25g of cuprous chloride, 10g of copper powder, 90g of sodium bicarbonate, and 900g of ethanol into a 3L autoclave, seal the autoclave, replace with nitrogen for three times, then pass 150g of liquid ammonia into the autoclave Then the temperature was raised to 200°C, the pressure was 10MPa, and the reaction was carried out at this temperature for 12h, and then the temperature was lowered to room temperature to remove insoluble solids by filtration. The resulting liquid was rectified under reduced pressure to obtain 158g of p-chlorobenzotrifluoride and 92g of p-trifluoromethylaniline.
Embodiment 3
[0031] Add 300g of p-chlorobenzotrifluoride, 2.5g of cuprous chloride, 0.5g of copper powder, 60g of sodium hydroxide, 600g of methanol to a 3L autoclave, seal the autoclave, and after nitrogen replacement for three times, pass 240g of it into the autoclave Liquid ammonia was then heated to 210°C, the pressure was 11MPa, and reacted at this temperature for 12h, then cooled to room temperature and filtered to remove insoluble solids. The resulting liquid was rectified under reduced pressure to obtain 155g of p-chlorobenzotrifluoride and 95g of p-trifluoromethyl aniline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com